Description
Channelrhodopsins are light-gated ion channels that serve as photoreceptors in photosynthetic microbes and have been applied as crucial optogenetic tools in genetic model organisms. When expressed in animals, they enable light-inducible control of ionic membrane permeability, which directly manipulates the activity of neurons expressing the protein. The application of channelrhodopsin-based optogenetics is particularly powerful when used in conjugation with the cGAL (GAL4-UAS) bipartite system (Wang et al., 2017). The mating of neuron-specific GAL4 driver lines to new channelrhodopsin effector lines could expand the genetic toolkit to perturb and manipulate neural circuits in the organism.
Blue light-gated channelrhodopsins have been widely used in Caenorhabditis elegans neurobiology but often have to be performed in lite-1(ce314) mutant backgrounds because short-wavelength blue light is an aversive cue in wild-type animals and directly affects C. elegans neuronal physiology. Previously, a red light-gated variant of channelrhodopsin, termed Chrimson, has been successfully applied in fruit fly and mice, and has recently been codon-optimized for use in C. elegans (Klapoetke et al., 2014; Schild and Glauser, 2015).
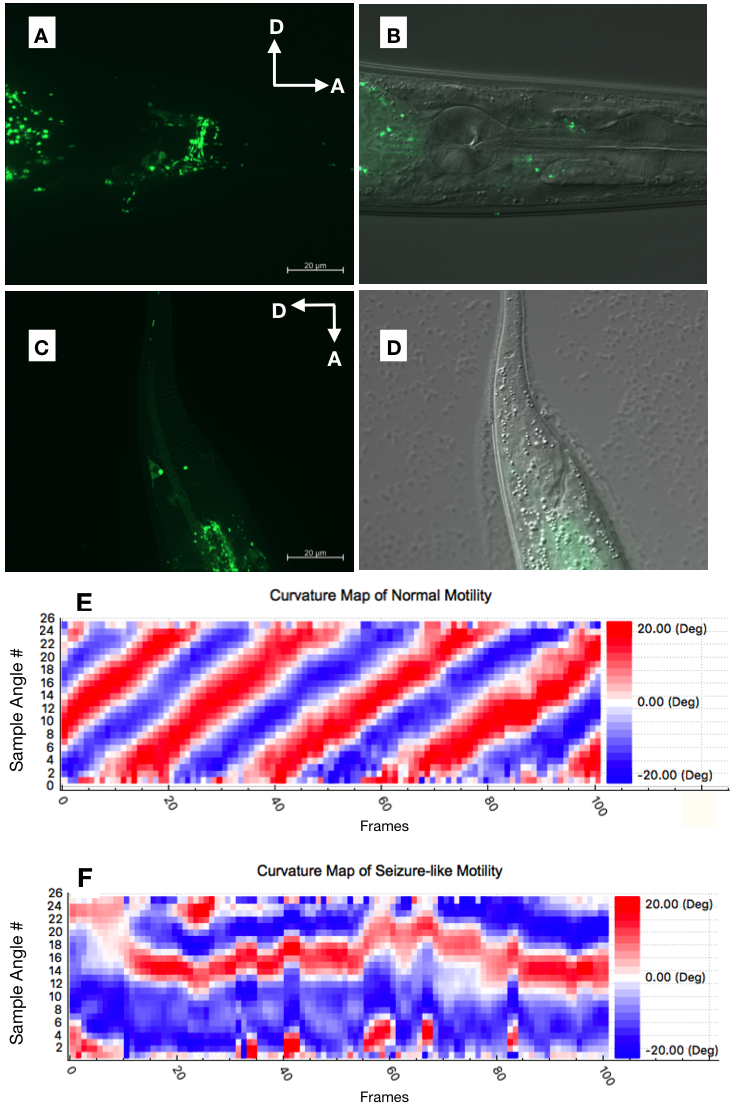
Here, we constructed a Chrimson (15xUAS::chrimson::gfp) cGAL effector line. We introduced the UAS::chrimson::gfp effector DNA construct as an extrachromosomal array into a previously published cGAL pan-neuronal driver line (PS6961 syIs334) and generated integrants on chromosome II (PS8023, syIs503) and chromosome V (PS8024, syIs504) (Table 1) via standard X-ray irradiation. We showed Chrimson-GFP expression in the C. elegans head and tail neurons (Fig. 1A-1D). We also showed that red light could induce a seizure-like motility phenotype in C. elegans expressing Chrimson-GFP in a pan-neuronal manner (videos), while the negative controls expressing only the effector, or without light induction showed regular motility as expected (Table 2). The body curvature maps from normal and seizure-like motilities showed distinct patterns (Fig. 1E and 1F). We report the effector construct of red-light-gated channelrhodopsin Chrimson as an addition to our cGAL toolkit, which could be widely used in future research to overcome the technical restrictions of blue light-gated channelrhodopsins in C. elegans.
Videos: Representative videos showing normal motility (video 1) and seizure-like motility (video 2) in a C. elegans adult hermaphrodite. Video 1: C. elegans co-expressing pan-neuronal driver and Chrimson-GFP effector (PS8026 syIs504 V; syIs334 X) was incubated with all-trans retinal (ATR) overnight and recorded motility without light stimulus, showing normal motility. Video 2: the same animal recorded in video 1 under the induction of intermittent red-light stimulus, showing seizure-like motility.
Table 1: A list of C. elegans strains used in this research.
| Strain | Genotype | Description | Outcrossed | Reference |
| PS6961 | syIs334 X | Integrated pan-neuronal driver (Prab-3) | 2x | Wang and Liu et al., 2017 |
| PS7900 | syEx1626; syIs334 X | Chrimson extrachromosomal array in Prab-3 driver background | NA | This research |
| PS7945 | syEx1626 | Chrimson extrachromosomal array in N2 background | NA | This research |
| PS7946 | syIs334 syIs497 X | Prab-3 driver and Chrimson effector | 4x | This research |
| PS8023 | syIs503 II | Chrimson effector | 3x | This research |
| PS8024 | syIs504 V | Chrimson effector integrated on LGV | 3x | This research |
| PS8025 | syIs503 II; syIs334 X | Chrimson effector; Prab-3 driver | 3x | This research |
| PS8026 | syIs504 V; syIs334 X | Chrimson effector; Prab-3 driver | 3x | This research |
| DA438 | bli-4(e937) I; rol6(e187) II; daf2(e1368) vab-7(e1562) III; unc-31(e928) IV; dpy-11(e224) V; lon2(e678) X | Mapping strain | NA | Avery, 1993 |
Table 2: Red light induces seizure-like motility exclusively in animals co-express cGAL pan-neuronal driver and Chrimson effector. The numbers in the parentheses indicate the total numbers of animals screened in the behavioral assay, out of which 100% of the animals showed the same phenotype under each condition. (see videos for normal and seizure-like motility).
| Driver | – | + | + | + |
| Effector | + | + | + | + |
| ATR | + | – | + | + |
| Light stimulus | + | + | – | + |
| syIs503 | Normal motility (11) | Normal motility (7) |
Normal motility (11) |
Seizure (10) |
| syIs504 | Normal motility (11) |
Normal motility (7) |
Normal motility (11) |
Seizure (11) |
Methods and Reagents
Molecular Cloning: pJL057(15xUAS::chrimson::gfp::let-858 3’UTR) was created by inserting Chrimson via Gibson assembly into pHW394 cut with Kpn-I (NEB) upstream of the GFP. Chrimson was isolated via PCR using oJL122 (5’ttgGCTAGCgtcgacGGTAccaaaaATGGCCGAACTCATCTCATCG3’) and oJL123 (5’ CCTTTACTCATtttttctaccGGGACTGTATCCTCATCTTCCTC3’) from the template plasmid dg263 (Addgene #66101).
DNA transformation in C. elegans: Transgenic animals were generated using standard microinjection techniques, as found in Mello et al. Extrachromosomal arrays were integrated in the genome via X-ray irradiation.
Strains: PS7900 syEx1626; syIs334 X was created by injecting pJL057(15xUAS::chrimson::gfp::let-858 3’UTR, 50 ng/uL), Pofm-1::gfp (50 ng/uL), and 150ng/uL of 1kb DNA ladder (NEB) into PS6961 (Prab-3::GAL4 driver strain).
PS7946 syIs334 syIs497 X was created by integrating PS7900 and outcrossed 4x.
PS8023 syIs503 II and PS8024 syIs504 V were obtained by integration of PS7945 syEx1626 and outcrossed.
Mapping: To map the effector alleles to one of the six linkage groups in C. elegans, we used previously published methods by mating the effector lines with DA438 line (Walton et al., 2017 and Avery 1993).
Microscopy and Fluorescence Screen: The pan-neuronal expression of Chrimson-GFP was imaged under 100X magnification using a Zeiss Imager Z2 with Apotome 2.0 using Zen BLUE 2.3. Adult hermaphroditic worms were paralyzed on agarose pad (containing 3 mM levamisole and 2% agarose in M9 buffer). GFP images in Fig. 1A and 1C are maximum intensity projections.
Optogenetics experiments: Escherichia coli strain OP50 was grown in LB at 37° C overnight with aeration. The all-trans retinal (ATR, Sigma) was resuspended in ethanol to 100 mM and diluted 1:200 with the OP50 overnight culture to a final concentration of 500 µM. 100 uL of mixture of ATR and bacteria were seeded onto each NGM plates and air-dried for more than 30 min prior to the experiment.
A total 12 animals from each line were picked onto three OP50 plates (4 animals per plate) with or without ATR and incubated overnight in the dark before the optogenetic experiments. The motility of worms was recorded for 2 min (7.5 frames per second) and analyzed using Wormlab Tracking system (MBF Biosciences). To induce depolarization, nematodes were exposed to pulses of red LED stimulus with 100 ms duration, 1100 ms interval length, and 100% intensity.
Acknowledgments
We thank Dr. Han Wang for his comments, suggestions, and support.
References
Funding
R21 MH115454/MH/NIMH NIH HHS/United States
Reviewed By
Brigitte Le BoufHistory
Received: August 7, 2018Accepted: August 21, 2018
Published: August 22, 2018
Copyright
© 2018 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Cao, M; Chai, C; Liu, J; Sternberg, PW (2018). Application of the red-shifted channel rhodopsin Chrimson for the Caenorhabditis elegans cGAL bipartite system. microPublication Biology. 10.17912/2JGW-FJ52.Download: RIS BibTeX