Description
Caenorhabditis elegans naturally thrives in a soil environment where they feed on bacteria and are in constant association with a diverse range of microbes (Barker et al. 1994). C. elegans egg laying is delayed or reduced when animals are infected with Burkholderia pseudomallei, Burkholderia thailandensis, Staphylococcus aureus, and Serratia marcescens (Mallo et al. 2002; O’Quinn et al. 2002; Irazoqui et al. 2010). These changes in egg laying may be a protective response to pathogenic bacteria. Mutants of TGF-β-like DBL-1 signaling pathway also display reduced brood size (Luo et al. 2009; Roberts et al. 2010). While the peak of egg-laying activity seen in normal animals between days two and four is depressed in dbl-1 pathway mutants, the reproductive span of these dbl-1 pathway mutants is increased to up to 13 days (Luo et al. 2009). To determine if the egg-laying observed during infection is DBL-1 pathway-dependent, we tested the effect of the DBL-1 signaling pathway on egg laying when C. elegans were fed on representative Gram-negative (S. marcescens) and Gram-positive bacteria (Staphylococcus epidermidis).
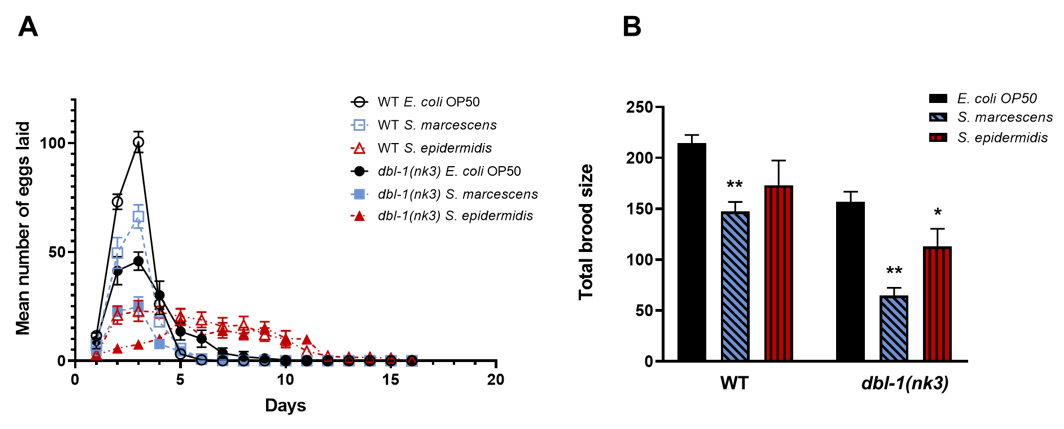
Similar to previously published reports, we found that loss of DBL-1 pathway signaling decreases brood size and increases reproductive span in normal laboratory conditions (E. coli strain OP50 and 20°C incubation) (Figure 1A and B) (Luo et al. 2009; Roberts et al. 2010).
Here, we report three new results. First, brood size reductions caused by infection and by loss of DBL-1 signaling are independent (Figure 1A). Wild-type and dbl-1(nk3) animals both significantly decrease their brood size when grown on S. marcescens (p= 0.005 and p< 0.001, respectively). dbl-1 mutant animals laid even fewer eggs than the wild-type animals on S. marcescens, suggesting that the reduced brood size phenotype is independently affected by both S. marcescens exposure and by loss of DBL-1 (p< 0.001). While the decrease in brood size of wild-type animals on S. epidermidis was not significant (p= 0.115), the decreased brood size of dbl-1(nk3) animals was significant on this pathogenic bacterial strain (p= 0.045). Indeed, the decreases in brood size upon infection with either S. marcescens or S. epidermidis in both wild-type and dbl-1(nk3) populations are similar (p= 0.57), suggesting the pathogenic bacteria affect brood size independent of DBL-1. Because dbl-1(nk3) populations display a further reduced brood size upon infection by either pathogen compared to the wild type, the negative effects of pathogen exposure and loss of DBL-1 signaling on brood size appear to be additive (p< 0.05).
Second, while the wild-type population on S. marcescens survived until all animals ceased laying eggs, all dbl-1(nk3) animals died on S. marcescens by Day 5. These results explain why the extended reproductive span normally seen in dbl-1(nk3) populations was not observed on S. marcescens. These results also support previous reports of decreased viability of dbl-1 mutant animals on another variety of S. marcescens, Db11 (Mallo et al. 2002).
Third, S. epidermidis affects egg-laying patterns similar to loss of dbl-1 function. Initially, both wild-type and dbl-1(nk3) strains on S. epidermidis have reduced eggs laid in the first four days compared to strains grown on the E. coli control. Loss of DBL-1 further reduced the number of eggs laid during each of these days, suggesting that this phenotype is independently affected by both S. epidermidis exposure and by loss of DBL-1 (p= 0.004). Then, both wild-type and dbl-1(nk3) strains on S. epidermidis have similar extended reproductive spans, extending to at least day 13 (one tenacious wild-type hermaphrodite laid embryos until day 15). This S. epidermidis-induced reproductive span extension appears to be independent of DBL-1 signaling, because the numbers of eggs laid by both wild-type and dbl-1(nk3) populations between days 5 and 16 were similar at these time points (p= 0.509).
Methods
Request a detailed protocolAnimals were age-synchronized by hypochlorite treatment (Stiernagle, 2006) and grown on plates seeded with Escherichia coli OP50. Ten L4 animals were manually transferred to individual plates seeded with S. marcescens or S. epidermidis. Plates were completely covered by bacteria to prevent animals from avoiding the bacteria. Adults were daily transferred to new plates and the number of eggs laid on each plate was counted every 24 hours until no more eggs were laid. Statistical analyses were performed using repeated measures ANOVA and Tukey’s post-hoc test.
Reagents
Strains were maintained on EZ media plates at 20°C (0.55 g Tris-Cl, 0.24 g Tris base, 3.1 g BD BactoTM Peptone, 8 mg cholesterol, 2 g sodium chloride, 20 g agar, in water to 1 L (E. Lambie, personal communication). The C. elegans strains used were N2 and NU3 dbl-1(nk3). The Gram-negative bacterial strains used were Escherichia coli OP50 (CGC) and Serratia marcescens (Carolina Biological Supply Company). The Gram-positive bacterial strain used was Staphylococcus epidermidis (ATCC 49134). S. marcescens and S. epidermidis were provided by A. J. Hammett, TWU. All bacterial strains were grown for 9 hours in tryptic soy broth at 37°C before plating on EZ media plates.
References
Funding
This work was supported by the TWU Department of Biology, Texas Woman's University, and USDA NIFA grant 2019-67031-28999. Dr. Paul Yeatts at the TWU Center for Research Design and Analysis provided statistical assistance.
Reviewed By
Cathy Savage-DunnHistory
Received: March 28, 2019Accepted: April 12, 2019
Published: April 15, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Madhu, BJ; Salazar, AE; Gumienny, TL (2019). Caenorhabditis elegans egg-laying and brood-size changes upon exposure to Serratia marcescens and Staphylococcus epidermidis are independent of DBL-1 signaling. microPublication Biology. 10.17912/2r51-b476.Download: RIS BibTeX