Description
The aging of an organism is heterogeneous, that is to say, tissues and organs may age at unique rates compared to overall organism longevity. Inhibition of the insulin/insulin-like growth factor signaling (IIS), such as the daf-2 IIS receptor increases Caenorhabditis elegans longevity. The daf-2 pathway plays a key role in the regulation of metabolism and stress responses and as such, impacts the longevity of the organism (Uno and Nishida 2016). Evidence suggests that an increase in insulin signaling results in a reduction in overall lifespan and a decrease in neuronal longevity (Kenyon 2010). Recently, the gbb-1 GABA signaling pathway has also been implicated in the regulation of adult longevity (Chun et al. 2015). The GABAergic motor neurons innervate the dorsal and ventral body muscles controlling locomotion, foraging and defecation of the animal. GABA, an inhibitory neurotransmitter, has been found to decrease the lifespan of the nematodes (Chun et al. 2015), however, its role in neuronal aging has yet to be determined. The formation of ectopic neurite branches and production of axonal beads are two known phenotypes of neuronal aging in C. elegans. In this study, we examined the aging of the GABAergic motor neuron nerve cords and dorsal commissures.
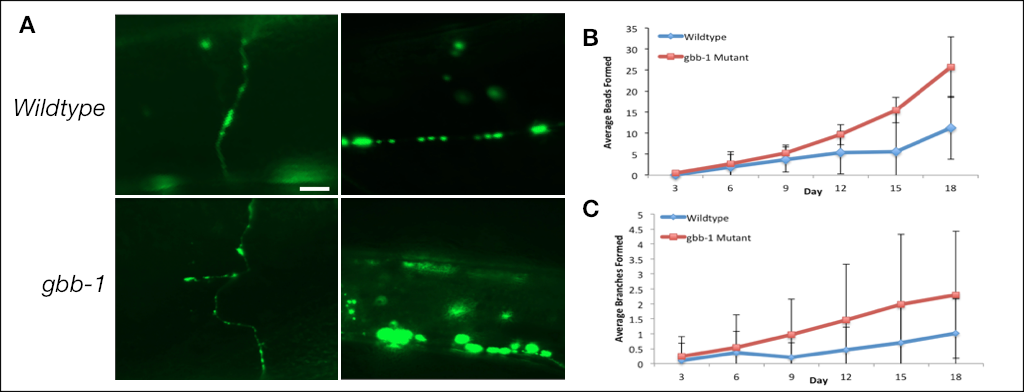
Fifty age-synchronized worms were imaged every three days (days 3, 6, 9, 12, 15, 18). Worms were plated on Fluorodeoxyuridine (FUdR) treated agar plates in order to maintain only adult worms throughout the imaging process (Mitchell et al. 1979). Neurons were visible using the GABAergic specific unc-25::GFP marker strain. To keep the data consistent, images were taken of the GABAergic axons and dorsal commissures between the VD5 and DD5 cell bodies (Panel A, scale bar 10 microns). Fluorescent images were taken using the Zeiss Axioplan fluorescent microscope under 40x objective. To prepare slides for fluorescence microscopy, an agarose immobilization protocol was followed (Fang-Yen et al. 2009). Aging of the neurons was quantified by counting the number of axonal beads and branches present on the neural processes. Branched processes emanating from the cell body, neural axon or dorsal commissures were scored as aging phenotypes. As predicted, GABAergic neurons displayed phenotypic evidence of aging.
The C. elegans genome encodes two metabotropic GABAB receptor genes, gbb-1 and gbb-2, which are homologous to their mammalian counterparts (Chun et al. 2015). GABA binds to the gbb-1 receptor initiating a signaling cascade that ultimately inhibits the daf-16 transcription factor. A reduction of function mutation in the gbb-1 encoded GABA receptor subunit increases worm lifespan; however, it seems to be specific to the GBB-1 subunit as no effect was observed in a loss of function gbb-2 mutation. When overexpressed, gbb-1 mutants were short-lived which is consistent with GABA signaling regulating lifespan (Chun et al. 2015). To determine the effect of GABA signaling on neuronal aging, we observed the effect of a loss-of-function gbb-1 mutant on the aging of the GABAergic motor neurons. Unexpectedly, the GABAergic dorsal commissures and axons in the gbb-1 mutants displayed a significant increase in aging morphology compared to the wild type (Panel A). gbb-1 mutants displayed a significant increase in beading (Panel B, Table 1) and branching (Panel C, Table 2) in the GABAergic motor neurons compared to the wild type from day 9 onwards (p<0.05, independent t-test, n=50). Thus gbb-1(tm1406) mutants although long lived, have neurons that appear to age faster. This work was done with a single gbb-1 allele, in the future it will be important to verify our findings with additional gbb-1 alleles or show that a transgene with the wildtype gbb-1 can suppress the observed aging phenotypes.
Table 1. Average bead formation on GABAergic dorsal commissures and axons between DD5 and VD5 cell bodies in N2 wild type and gbb-1 mutant worms (n=50).
| Day | 3 | 6 | 9 | 12 | 15 | 18 |
| wild type | 0.06 ± 0.24 | 1.88 ± 2.87 | 3.70 ± 1.94 | 5.36 ± 2.42 | 5.56 ± 3.01 | 11.26 ± 7.23 |
| gbb-1 mutant | 0.50 ± 1.05 | 2.7 ± 3.01 | 5.24 ± 2.95 | 9.62 ± 5.06 | 15.46 ± 9.43 | 25.7 ± 7.49 |
| p-value | 0.005* | 0.166 | 0.003* | 5.27 x 10-7* | 2.26 x 10-10* | 3.12 x 10-16* |
Table 2. Average branch formation on GABAergic dorsal commissures and axons between DD5 and VD5 cell bodies in N2 wild type and gbb-1 mutant worms (n=50).
| Day | 3 | 6 | 9 | 12 | 15 | 18 |
| wild type | 0.10 ± 0.58 | 0.36 ± 0.72 | 0.2 ± 0.49 | 0.46 ± 0.76 | 0.7 ± 1.23 | 1.02 ± 1.15 |
| gbb-1 mutant | 0.24 ± 0.66 | 0.54 ± 1.09 | 0.96 ± 1.19 | 1.46 ± 1.86 | 1.98 ± 2.34 | 2.3 ± 2.13 |
| p-value | 0.261 | 0.333 | 6.93 x 10-5* | 6.38 x 10-4* | 9.19 x 10-4* | 3.13 x 10-4* |
Reagents
The strains used in this study were: CZ10175: zdIs5[Pmec-4::GFP], IC2142: zdIs5[Pmec-4::GFP]; gbb- 1(tm1406), CZ1197: juIs73[Punc-25::GFP], IC2141: juIs73[Punc-25::GFP]; gbb-1(tm1406).
References
Funding
This project was supported by the Canadian Institutes of Health Research (CIHR) and the National Sciences and Engineering Council of Canada (NSERC).
Reviewed By
AnonymousHistory
Received: May 29, 2018Accepted: September 3, 2018
Published: September 6, 2018
Copyright
© 2018 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Dhillon, I; Momin, A; Chin-Sang, I (2018). GABA Receptor mutant gbb-1 accelerates morphological aging of GABA neurons in Caenorhabditis elegans. microPublication Biology. 10.17912/D7E5-WJ67.Download: RIS BibTeX