Description
Several techniques are available for spatiotemporal control of genome recombination and gene expression in the nematode Caenorhabditis elegans. Here we report a novel tool to combine the powerful FLP-Frt and GAL4-UAS systems to increase their versality and to offer additional levels of control.
FLP is an enzyme that catalyzes recombination between two short Frt DNA sequences and is frequently used to excise genomic fragments flanked by Frt sites, thereby either activating or knocking out gene expression, depending on the experimental design (Hubbard 2014). Recently, we generated multiple strains that stably express FLP in different somatic tissues from single-copy transgenes and demonstrated that they in most cases induce recombination in ~100% of the cells of the expected tissue (Munoz-Jimenez et al. 2017). We subsequently constructed a strain for germline recombination to permanently knock out Frt-flanked genes or exons (Macías-León and Askjaer 2018).
The GAL4-UAS system is based on the Saccharomyces cerevisiae Gal4p transcription factor and its cognate DNA target called upstream activating sequence (UAS). Typically, this bipartite system includes a series of ‘driver’ strains expressing GAL4 in specific tissues and one or several strains with an ‘effector’ gene downstream of UAS repeats. Wang and colleagues from the Sternberg laboratory recently optimized the GAL4-UAS system for C. elegans (“cGAL”) and reported several tissue-specific cGAL drivers (Wang et al. 2017). Moreover, they have developed a split cGAL toolkit where the DNA binding and activation domains are expressed as individual polypeptides, thereby enabling further fine-tuning of spatiotemporal control: only when and where the two components are co-expressed they will activate the UAS::effector transgene (Wang et al. 2018).
We reasoned that a UAS::FLP effector strain would be a useful addition to the C. elegans genome engineering and gene expression toolbox. In particular, it will enable 1) recombination in tissues for which cGAL drivers have been generated, without the need to create new FLP drivers and 2) the use of intersectional promotors and split cGAL to induce recombination in cell types for which a specific promoter is not available. As proof of principle, we tested the ability of cGAL expressed in the intestine to induce UAS::FLP-mediated genome recombination.
To generate a UAS::FLP effector strain, we first excised a 15xUAS::∆pes-10 fragment from pHW394 (Wang et al. 2017; kind gift from Han Wang and Paul Sternberg) by digestion with HindIII (blunted by Klenow) and KpnI and inserted it into pBN338 (Munoz-Jimenez et al. 2017) digested by NotI (blunted by Klenow) and KpnI. This generated plasmid pBN449 UAS::FLP::SL2::mNG, which contains homology arms for Mos1-mediated insertion into the cxTi10882 locus on chr IV of strain EG6700. We employed standard MosSCI procedures (Frøkjær-Jensen et al. 2012) to obtain the integrated strain BN908 (see strain genotypes in Reagents below). We next crossed BN908 and PS6935 (from CGC) to BN596 to obtain BN923 and BN918, respectively. Finally, BN908 was crossed to BN918 to obtain BN919.
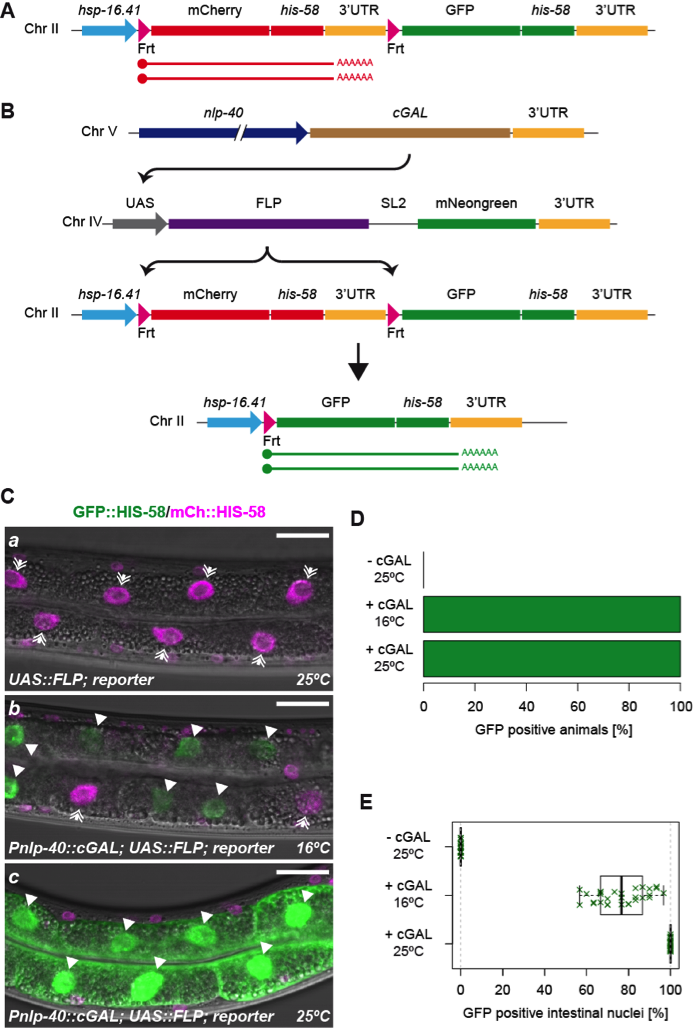
As readout for FLP activity, we used a heat-inducible dual color reporter (Fig 1A; Munoz-Jimenez et al. 2017). In the absence of cGAL (strain BN923), the reporter produces only mCherry::HIS-58, demonstrating that spurious expression from the UAS::FLP is not detectable (Fig 1Ca; Fig 1D; >100 animals analyzed at 16°C and 25°C). When combined with cGAL expressed in the intestine from a nlp-40 promoter (strain BN919), bright nuclear GFP::HIS-58 was detected specifically in this tissue, indicative of correct FLP activation and excision of the mCherry::his-58 cassette (Fig 1B; Fig 1Cb-c). GFP::HIS-58 expression was observed in all animals both at 16°C and 25°C (Fig 1D; >100 mixed stage animals in each condition). Moreover, in animals grown at 25°C, recombination was detected in 100% of the intestinal cells (Fig 1Cc; Fig 1E; n=24 L2-L4 larvae), arguing that the combined activity of the UAS-GAL4 and FLP-Frt systems is very high. In animals grown at 16°C, the recombination efficiency was slightly lower with an average of 76.4% of the intestinal cells expressing GFP::HIS-58 (Fig 1Cb; Fig 1E; n=29 L2-L4 larvae; standard deviation = 11.7%). We propose that the temperature dependency is mainly due the characteristics of cGAL because 1) the original description of the cGAL system reported lower activity at 16°C vs 25°C (Wang et al. 2017), 2) we observed lower expression of cytoplasmic mNeongreen at 16°C vs 25°C (co-expressed from the FLP transgene; compare Fig 1Cb-c) and 3) direct expression of FLP from an elt-2 promoter induces recombination in ~100% of intestinal cells at 16°C (Munoz-Jimenez et al. 2017). The lower activity at 16°C can potentially be overcome by producing effector strains with multiple copies of the UAS::FLP construct (Han Wang, personal communication).
In conclusion, we have developed a novel tool that enables combining the highly efficient UAS-GAL4 and FLP-Frt systems to control genome recombination and gene expression with more versatility and better precision. We provide the UAS::FLP effector strain to the community for use with existing and future cGAL drivers.
Reagents
Plasmid pBN449 UAS::FLP::SL2::mNG.
Strain BN596 bqSi294[pBN154(unc-119(+) Phsp-16.41::FRT::mCh::his-58::FRT::gfp::his-58)] II; bqSi577[pBN306(unc-119(+) Pmyo-2::GFP)] IV. Available from the authors; however, if the dual color bqSi294 effector is not needed, we recommend using BN578, which carries the same visible marker in the MosSCI locus on chr IV (cxTi10882) and an additional visible marker on chr II (ttTi5605). BN578 is available at the CGC and facilitates crosses involving MosSCI strains that carry non-visible insertions in the ttTi5605 and cxTi10882 sites.
Strain BN908 unc-119(ed9) III; bqSi908[pBN449(unc-119(+) UAS::FLP::SL2::mNG)] IV. Will be available at the CGC pending CGC approval.
Strain BN918 bqSi294[pBN154(unc-119(+) Phsp-16.41::FRT::mCh::his-58::FRT::gfp::his-58)] II; bqSi577[pBN306(unc-119(+) Pmyo-2::GFP)] IV; syIs320[Pnlp-40::NLS::GAL4SK::VP64 + Pmyo-2::NLS::mCh] V. Available from the authors.
Strain BN919 bqSi294[pBN154(unc-119(+) Phsp-16.41::FRT::mCh::his-58::FRT::gfp::his-58)] II; bqSi908[pBN449(unc-119(+) UAS::FLP::SL2::mNG)] IV; syIs320[Pnlp-40::NLS::GAL4SK::VP64 + Pmyo-2::NLS::mCh] V. Available from the authors.
Strain BN923 bqSi294[pBN154(unc-119(+) Phsp-16.41::FRT::mCh::his-58::FRT::gfp::his-58)] II; bqSi908[pBN449(unc-119(+) UAS::FLP::SL2::mNG)] IV. Available from the authors.
References
Funding
This work was funded by the Spanish Ministry of Economy and Competitiveness (BFU2016-79313-P).
Reviewed By
Jordan WardHistory
Received: January 25, 2019Accepted: January 28, 2019
Published: January 29, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Ayuso, C; Askjaer, P (2019). Spatiotemporal control of genome recombination through combined FLP-Frt and GAL4-UAS technologies. microPublication Biology. 10.17912/micropub.biology.000089.Download: RIS BibTeX