Description
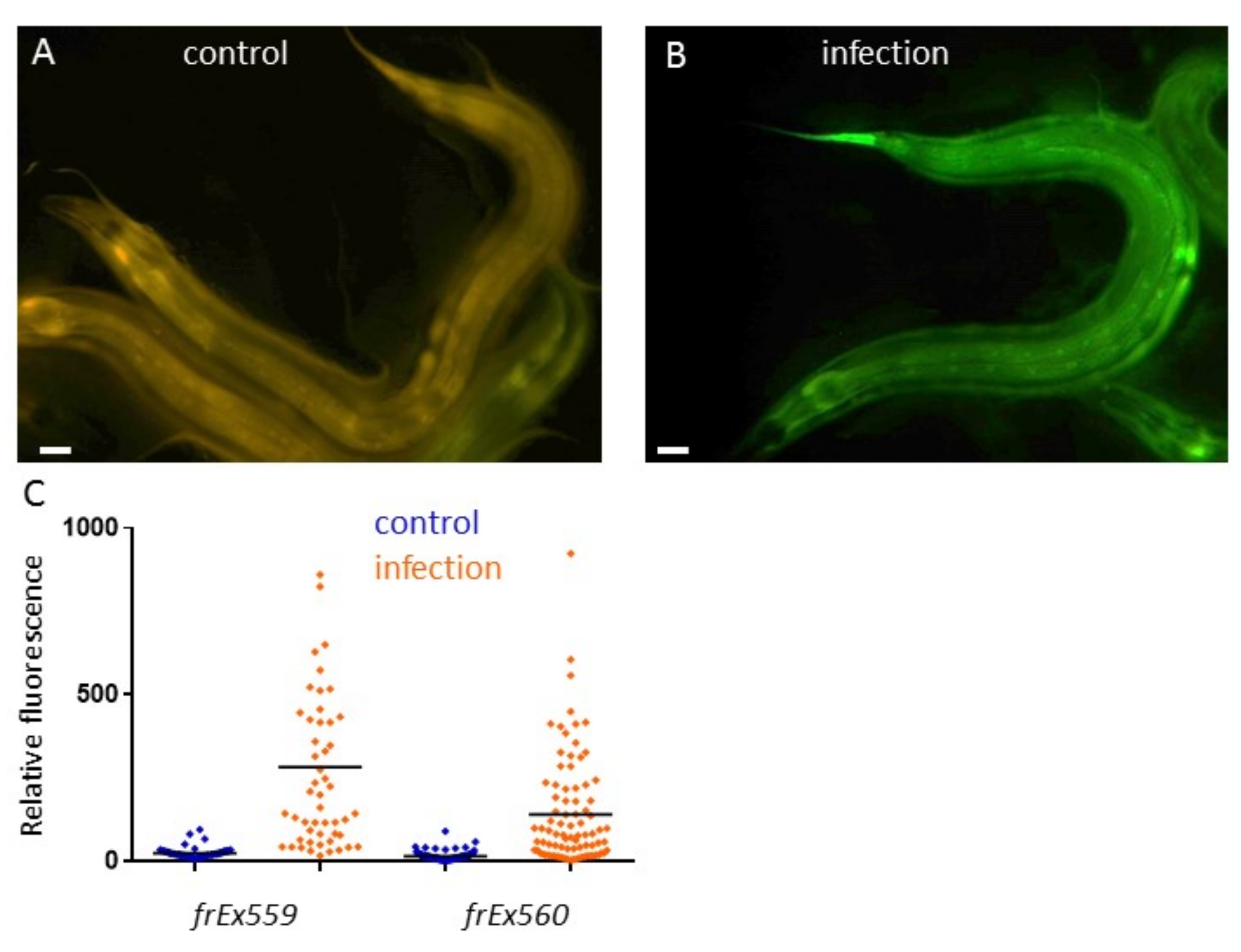
Inducible immune responses are ubiquitous features of animal defences against infection. D. coniospora is a natural pathogen of nematode. It produces spore that attach to specific glycans on the worm’s cuticle surface coat (Rouger et al., 2014), pierce it and send hyphae throughout the organism. This triggers the rapid induction in the epidermis of genes from the nlp (for neuro-peptide-like protein) and cnc (caenacin) families. These genes encode structurally-related antimicrobial peptides (AMPs) (Pujol et al., 2012); their over-expression can lead to an increased resistance to infection are likely to be important in nature for the survival of worms (Taffoni and Pujol, 2015). Other genes are up-regulated after infection, including F48C1.9 encoding for a nematode specific small secreted peptide (Pujol et al., 2008b). To monitor its expression, we made transgenic strains containing the transcriptional reporter F48C1.9p::GFP with the co-injection marker col-12p::DsRed, which is constitutively expressed in the epidermis (Pujol et al., 2008a). Upon infection with D. coniospora, an induction of the GFP was observed in the epidermis of the worm (Figure 1). This work suggest that another uncharacterized peptide encoding gene is part of the immune response of the worm epidermis.
Reagents
Constructs: F48C1.9p::GFP was obtained by Gibson fusion of 1.1 kb of the F48C1.9 promoter in pPD95.75 amplified with primers CACAACGATGGATACGCTAAaatcaaattatgacgtgatgcc and GTTCTTCTCCTTTACTCATgtttgttgaagatctgatctg. Injected at 80 ng/µl together with col-12p::DsRed (Pujol et al, 2008a) at 20 ng/µl. Two independent transgenic strains were obtained IG1514 frEx559 and IG1515 frEx560[F48C1.9p::GFP; col-12p::DsRed.].
Acknowledgments
J Belougne for worm sorting, at the French National Functional Genomics platform, supported by the GIS IBiSA and Labex INFORM.
References
Funding
ANR-16-CE15-0001-01
Reviewed By
AnonymousHistory
Received: January 24, 2019Accepted: February 7, 2019
Published: February 8, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Omi, S; Pujol, N (2019). Inducible expression of F48C1.9 encoding a nematode specific secreted peptide in the adult epidermis upon Drechmeria fungal infection. microPublication Biology. 10.17912/micropub.biology.000090.Download: RIS BibTeX