Department of Biology, University of Miami, Coral Gables, FL 33146
Present address: Department of Neurology and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, MD 21205
Description
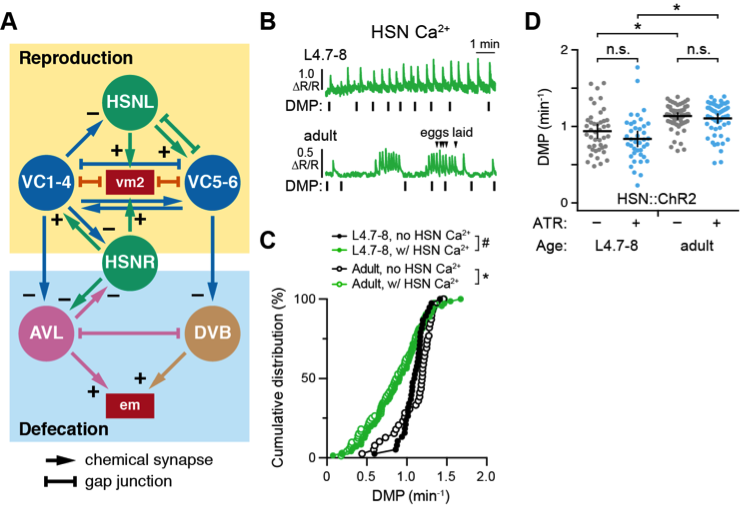
We have recently described an unusual rhythmic Ca2+ activity rhythm in the developing Hermaphrodite Specific Neurons (Ravi et al. 2018b). This ~50 s rhythm of HSN activity in L4.9 animals resembled the rhythm of the defecation motor program (DMP), prompting us to investigate whether there is a relationship between circuits that regulate reproduction and defecation behaviors. As shown in Figure 1A, the egg-laying HSN command neurons and VC motor neurons make and receive synapses from the excitatory GABAergic AVL and DVB motoneurons that regulate defecation (White, J.G. et al. 1986). Serotonin and Gαo signaling, which regulate egg laying behavior, can also signal to inhibit defecation (Ségalat et al. 1995; Waggoner et al. 1998; Hardaker et al. 2001; Tanis et al. 2008; Brewer et al. 2019). However, the functional relationship between what are thought to be independent motor circuits has not been examined. Because evidence shows that both the egg-laying active state and the DMP are both linked to changes in forward and reverse locomotion (Hardaker et al. 2001; Nagy et al. 2015), we reasoned there may be a similar relationship between expulsive behaviors that drive either egg laying or defecation.
Using a transgene that co-expresses GCaMP5 and mCherry in the HSNs from the nlp-3 promoter (Collins et al. 2016), we performed ratiometric Ca2+ imaging in L4.7-8 juveniles and egg-laying adults and compared the timing of HSN Ca2+ transients and defecation events (Ravi et al. 2018a). We found that defecation intervals in L4.7-8 and adult animals were significantly longer when they were accompanied by one or more HSN Ca2+ transients (Fig. 1B and 1C). This suggested the HSNs might signal to inhibit the defecation motor rhythm. To test this, we used a transgene that expressed Channelrhodopsin-2 in the HSNs from the egl-6 promoter (Emtage et al. 2012) and tested whether acute optogenetic activation of the HSNs in L4.7-8 juveniles or adults affected the DMP rhythm. Blue light illumination of animals grown on ATR, an essential cofactor for ChR2, caused a mild reduction in DMP frequency in L4.7-8 animals, but this effect was not statistically significant (p=0.2102) and was not observed in adults (Fig. 1D). Interestingly, we observed that DMP frequency was significantly longer in 24-hour adult animals compared to L4.7-8 juveniles (Fig. 1D). Previous work has shown a significant decline in the DMP frequency in aging animals, although this is reduction was not apparently related to changes in feeding as measured by pharyngeal pumping (Croll et al. 1977; Bolanowski et al. 1981). We propose that these changes in defecation frequency may relate to onset of egg-laying behavior and/or continued growth of adult animals.
Reagents
Strains are available from CGC: LX2004 vsIs183 [nlp-3p::GCaMP5::nlp-3 3’UTR + nlp-3p::mCherry::nlp-3 3’UTR + lin-15(+)] lite-1(ce314) lin-15(n765ts) X; LX1836 wzIs30 [egl-6::ChR2-YFP::unc-54 3’UTR + lin-15(+)] IV, lite-1(ce314) lin-15(n765ts) X; All-trans retinal (100 mM stock in ethanol) was from Sigma and added to pre-warmed OP50 bacterial cultures in B Broth as described (Collins et al. 2016; Ravi et al. 2018b). DMP frequency was measured based on the timing of the final expulsion step (Liu and Thomas 1994). Ratiometric Ca2+ imaging was performed in freely behaving animals as previously described (Collins et al. 2016; Ravi et al. 2018a; b).
References
Funding
The authors acknowledge support from NIH grant R01-NS086932.
Reviewed By
Niels RingstadHistory
Received: March 23, 2019Accepted: March 28, 2019
Published: March 29, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Ravi, B; Collins, KM (2019). Ca2+ activity in the HSN egg-laying command neurons and animal age is accompanied by a delay in the defecation motor program in Caenorhabditis elegans (I). microPublication Biology. 10.17912/micropub.biology.000093.Download: RIS BibTeX