Description
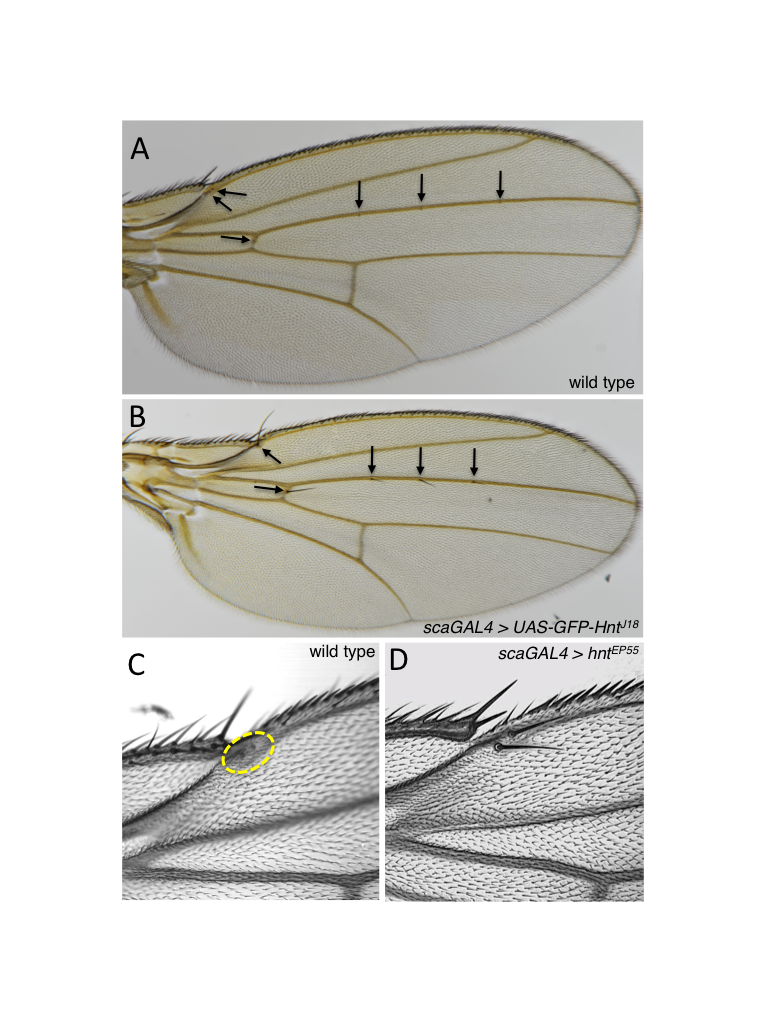
The adult Drosophila wing blade contains sense organs known as campaniform sensilla. These cellular structures sense pressure or strain in the wing during flight and provide neural feedback required for coordinated wing movements (Bartussek and Lehmann, 2016). The dorsal surface of the wing blade includes three campaniform sensilla along wing vein L3, two on the first longitudinal vein near the tip of the costa, and a single sensillum on the anterior cross vein. Other campaniform sensilla are found at the base of the wing and on the ventral wing surface (Huang et al., 1991). In experiments utilizing GAL4/UAS inducible expression of the Zinc finger transcription factor hindsight (hnt), also known as pebbled (peb) (Yip et al., 1997), we found that campaniform sensilla are frequently transformed to mechanosenory external sense organs, known as microchaetae. The GAL4 driver used was scaGAL4, a reporter driving expression of GAL4 in the pattern of the proneural gene scabrous (sca), which is expressed in sensory organ precursor cells as is hnt (Buffin and Gho, 2010). We found campaniform to microchaetae transformation occurred in the context of scaGAL4 driving expression of a particular transgene insertion, UAS-GFP-hnt[J18]. In order to rule out the possibility that this transformation was somehow specific to UAS-GFP-hnt[J18], we also tested scaGAL4 > peb[EP55] and found the same transformation. The penetrance of this phenotype was not complete, as not all campaniform sensilla were transformed to microchaetae in all individuals. Approximately half of progeny carrying scaGAL4 and either peb[EP55] or UAS-GFP-hnt[J18] display at least one transformation. To the best of our knowledge, there are two instances of this particular phenotype reported in the literature. The first is a specific allelic combination of loss-of-function mutants of the gene absent, small, or homeotic discs 2 (ash2[7]/ash2[18]) (Adamson and Shearn, 1996). The second involves the expression throughout the wing imaginal disc of a human SMAD tumour allele (UAS-SMAD4[100T]) (Takaesu et al., 2005). The former instance involving ash2 loss-of-function mutants is of particular interest because ash2 is implicated in the negative regulation of EGFR/Ras/MAPK signalling, through the negative regulation of rhomboid (Angulo et al., 2004), which is required for the production of active EGFR ligand (Wasserman et al., 2000). Thus, overexpression of hnt is consistent with overactivation of EGFR/Ras/MAPK signalling in the context of campaniform sensilla SOPs. Interestingly, hnt is the Drosophila homologue of human Ras Responsive Element Binding protein-1 (RREB-1) (Melani et al., 2008; Ming et al., 2013).
Reagents
The scaGAL4 (y w[*]; P{w[+mW.hs]=GawB}sca[109-68]/CyO) and peb[EP55] (w[1118] P{w[+mC]=EP}peb[EP55]) lines are available from the Bloomington Drosophila Stock Center (Bloomington stocks 6479 and 5358, respectively). The UAS-GFP-hnt[J18] line was recovered as follows: the original UAS-GFP-hnt insertion (Ming et al., 2013), which maps to the second chromosome, was mobilized by crossing to a Delta2-3 P element transposase stock (y w; ry[506] Sb P{ry[+t7.2]=Delta2-3}99B/TM6). F1 progeny were subsequently crossed to y w[1118]. Single F2 males lacking the Sb Delta2-3 chromosome and whose eye color differed substantially from that of the original UAS-GFP-hnt line, were isolated and propagated as stable stocks. Insertions mapping to the second or third chromosome were maintained as balanced or homozygous stocks and tested for GFP-hnt expression by crossing to the eye specific GMR-GAL4 driver (P{w[+mC]=GAL4-ninaE.GMR}12, Bloomington stock 1104). While the original UAS-GFP-hnt line was pupal lethal with GMR-GAL4, UAS-GFP-hnt[J18] produced viable progeny with a rough eye phenotype.
Wings were removed from anesthetized flies and placed in a drop of xylene on a microscope slide. Most xylene was then removed by touching the corner of a folded piece of tissue paper to the drop of xylene, leaving the wings in a thin film of xylene. A drop of Permount mountant (Fisher Scientific) was placed over the wings and a coverslip was placed over the wings in the mountant. Color wing micrographs were taken using a Nikon SMZ25 stereomicroscope equipped with Nikon Digital Sight Ri2 16.25MP color camera and processed using extended-depth-of-focus feature of Nikon NIS-Elements Arv4.50 software. The black and white images were obtained using a Nikon Eclipse 90i fitted with a Nikon D-eclipse C1 scan head using Nikon EZ-C1 software and brightfield detection. Brightfield Z stacks were edited and assembled using ImageJ (Schneider et al., 2012) and an extended-depth-of-focus plugin.
References
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) through a Discovery Grant to BHR.
Reviewed By
AnonymousHistory
Received: March 30, 2019Accepted: May 16, 2019
Published: May 20, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Szablewski, K; Reed, BH (2019). Overexpression of hindsight in sensory organ precursors is associated with a transformation of campaniform sensilla to microchaetae in the Drosophila wing. microPublication Biology. 10.17912/micropub.biology.000103.Download: RIS BibTeX