Description
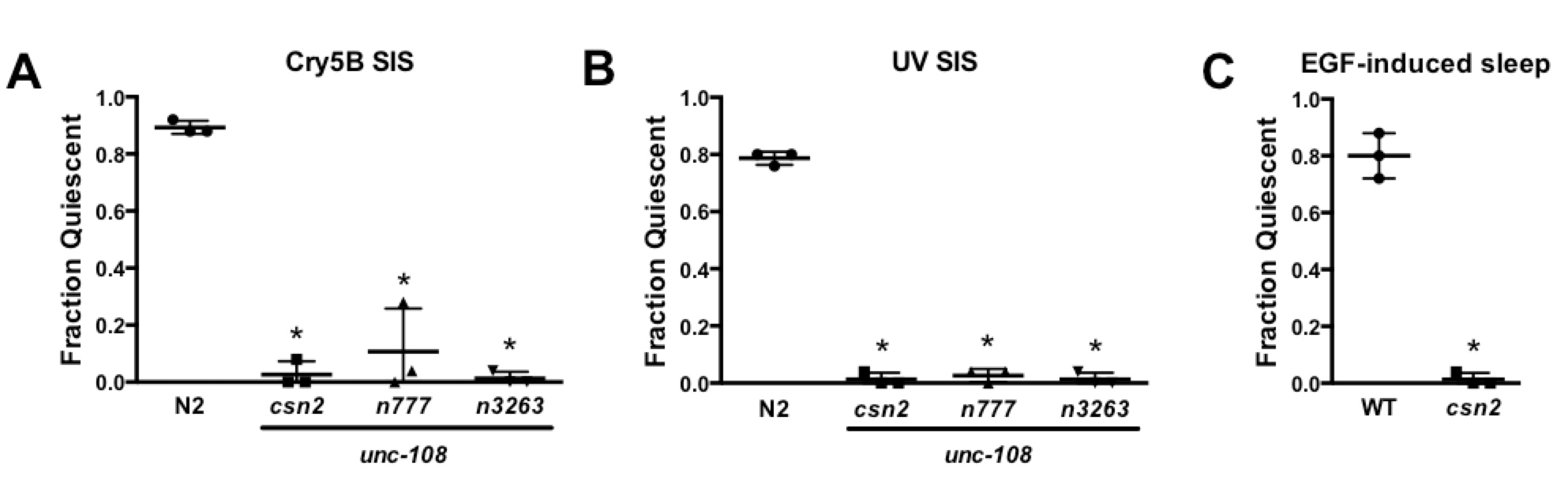
In a genetic screen for mutants defective in stress-induced sleep (SIS) we isolated csn2, an allele of the UNC-108/RAB-2 GTPase. The point mutation in unc-108(csn2) is identical to that in the previously characterized loss-of-function allele unc-108(n3263), substituting a glutamine in place of a glycine that is conserved among Ras superfamily members (Mangahas et al., 2008). Similar to other unc-108(lf) mutants, csn2 animals move slowly (not shown). Here we show that unc-108(csn2) as well as previously characterized unc-108 alleles are SIS-defective (Panels A, B). While the majority of wild-type N2 animals cease head movement, locomotion and pharyngeal pumping following exposure to damaging conditions, unc-108(lf) animals retain all of these activities. This coordinated impairment of sleep-associated behaviors argues against a role for UNC-108 downstream of the SIS-promoting ALA neuron, which acts via the collective action of neuropeptides with overlapping but distinct effects on the sub-behaviors of sleep (Nath et al., 2016).
SIS is dependent on Epidermal Growth Factor Receptor (EGFR) activation within ALA, and sleep can be triggered not only by noxious conditions but also by forced overexpression of LIN-3/EGF (Van Buskirk and Sternberg, 2007). We found that unc-108(csn2) animals are resistant to EGF-induced sleep (Panel C), indicating that UNC-108 functions downstream of EGF signaling within the SIS pathway. Together these results suggest that UNC-108 functions within ALA.
UNC-108 is widely expressed within the C. elegans nervous system and is implicated in the recycling of receptors through the endocytic pathway (Chun et al., 2008) as well as in dense core vesicle (DCV) maturation (Sumakovic et al., 2009; Edwards et al., 2009). We speculate that the UNC-108 SIS defect arises from deficits in EGFR trafficking or in the maturation of DCVs within the ALA neuron.
Reagents
Strains available from the CGC: N2 Bristol, MT1656unc-108(n777), ZH382 unc-108(n3263), PS5970 syIs197[hs::LIN-3c(cDNA) + myo-2p::DsRed + pha-1(+)];him-5. Strains available upon request: CVB30 unc-108(csn2), CVB31syIs197;unc-108(csn2).
References
Funding
This work was supported by an NSF Faculty Early Career Development Program (CAREER) award IOS#1553673 to CVB. Strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Reviewed By
David RaizenHistory
Received: April 23, 2019Accepted: April 26, 2019
Published: April 26, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Robinson, B; Van Buskirk, C (2019). UNC-108/RAB-2 is required for C. elegans stress-induced sleep. microPublication Biology. 10.17912/micropub.biology.000112.Download: RIS BibTeX