Description
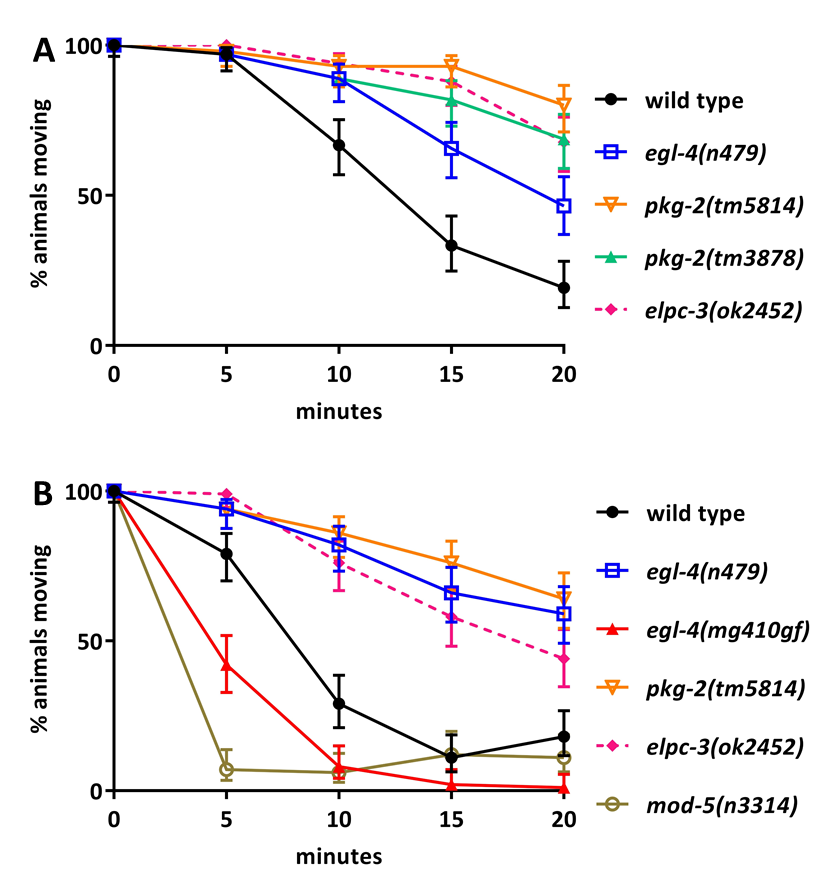
The C. elegans protein kinase G ortholog, EGL-4, has been demonstrated to be involved in C. elegans egg laying and the initiation of dwelling, a behavioral state characterized in part by low rates of locomotion (Trent et al. 1983; Hao et al. 2011). Serotonin regulates C. elegans egg laying through all five identified serotonin receptors, and serotonin promotes the dwelling state through the mod-1 serotonin receptor (Trent et al. 1983; Hapiak et al. 2009; Flavell et al. 2013; Brewer et al. 2019). Given that serotonin plays a role in both the egg laying and dwelling behaviors that egl-4 regulates, we sought to determine if protein kinase G is required for C. elegans to respond to serotonin. Exogenous serotonin paralyzes wild-type C. elegans (Gürel et al. 2012). The assays shown in Figure 1A indicate that one loss-of-function mutant of egl-4 and two independent loss-of-function mutants of the egl-4 paralog, pkg-2 are resistant to paralysis by serotonin, similar to the previously-known serotonin resistant mutant elpc-3(ok2452) (Gürel et al. 2012).The pkg-2(tm3878)and pkg-2(tm5814) alleles each carry deletions of sequences coding large portions of the conserved catalytic cGMP-dependent protein kinase domain of the PKG-2 protein, including the active site and ATP binding site (Hofmann et al. 1992), and thus we predict them to be null alleles. The egl-4(n479)mutation is an early stop codon prior to the kinase domain and n479 is thus also predicted to be a null allele (Fujiwara et al. 2002). The Mak lab isolated an egl-4 gain-of-function allele mg410 that gives rise to a K162N single amino acid substitution (Hao et al. 2011). This K162N mutation lies in the conserved pseudo-substrate motif of EGL-4, and is predicted to result in auto-phosphorylation even in the absence of cGMP, leading to constitutively active EGL-4 (Hao et al. 2011). Figure 1B demonstrates that this egl-4 gain-of-function mutant is hypersensitive to paralysis by serotonin, like the previously-known serotonin hypersensitive mutant mod-5(n3314) (Gürel et al. 2012), and in contrast to the serotonin-resistant egl-4 and pkg-2 loss-of-function mutants. Taken together these data indicate that the protein kinase G, egl-4, and its paralog pkg-2 mediate serotonin-induced paralysis of C. elegans.
How do the protein kinase G orthologs, EGL-4 and PKG-2, mediate serotonin signaling in C. elegans? Previously our lab performed a forward genetic screen to identify proteins involved in serotonin signaling (Gürel et al. 2012). The proteins identified includedtwo serotonin receptors, SER-4 and MOD-1, and several of the other proteins were predicted to act in the SER-4 or MOD-1 pathways. SER-4 is a G protein coupled receptor (Olde and Mccombie 1997) and MOD-1 is a serotonin-gated chloride channel (Ranganathan et al. 2000). It is possible that protein kinase G is acting in the SER-4 or MOD-1 pathways to control the effects of serotonin on C. elegans locomotion. Alternatively, protein kinase G could act with the MOD-5 serotonin transporter (SERT). Prior work indicates that phosphorylation of mammalian SERT increases its activity and that protein kinase G acts in a pathway to stimulate SERT, although protein kinase G may not directly phosphorylate SERT (Miller and Hoffman 1994; Kilic et al. 2003; Ramamoorthy et al. 2007; Wong et al. 2012; Zhang et al. 2016). However, additional studies indicate that stimulation of cGMP pathways reduces SERT activity in certain cell types (Pogun et al. 1994; Asano et al. 1997). It is possible that C. elegans protein kinase G negatively regulates MOD-5 function.
Methods
Request a detailed protocolAssays were performed by filling microtiter wells with 50 µl of M9 buffer (Sulston and Hodgkin 1988). 10 animals were picked to each well. Prior to addition of serotonin, the number of animals moving in each well was counted to generate the zero time point (in all cases 100% of the population was moving at time zero). 50 µl of 20 mM serotonin in M9 buffer was then added to each well, so that assays were carried out at a final concentration of 10 mM serotonin. To dissolve serotonin in M9 buffer, the M9 buffer was first heated in a water bath to 90°C prior to the addition of 5-hydroxytryptamine creatine sulfate. The serotonin solution was then allowed to return to room temperature before use in assays. Wells were scored under a dissecting microscope for the number of moving animals at 5, 10, 15 and 20 minutes after the addition of serotonin. “Moving” was defined as having smooth swimming movements of the entire body. Animals showing only movements of the head or only stiff or jerky movements of ≤50% of the body were scored as not “moving.” 100 animals total were assayed for each genotype. Error bars are 95% confidence intervals calculated in Prism v.7.01 as part of a contingency table analysis using the Wilson/Brown method.
The elpc-3(ok2452) and mod-5(n3314) alleles were included as controls; they were previously shown to be respectively resistant and hypersensitive to paralysis by exogenous serotonin (Gürel et al. 2012). In this assay it is critical to test any two strains to be compared in parallel, as results vary somewhat for the same strain assayed from day to day. For example, in Figure 1A, at 15 minutes 33% of the wild-type population is still moving, while in Figure 1B, only 11% of the wild-type population is still moving at the same time point. Despite this experiment-to-experiment variability, certain mutants are always more resistant or more sensitive to serotonin than is the wild type. For example, the egl-4 loss-of-function mutant is always more resistant to paralysis than is the wild type. In practice this means one can compare different strains within a single experiment, but cannot compare results from one experiment to the next.
Reagents
5-hydroxytryptamine creatine sulfate complex; Sigma H-7752
The wild-type strain was Bristol N2. Strains available from Caenorhabditis Genetics Center are N2, MT1074 egl-4(n479) IV, and MT9772 mod-5(n3314) I. Strains available upon request are FX3878 pkg-2(tm3878) IV, FX5814 pkg-2(tm5814) IV, LX1769 elpc-3(ok2452) V, and VS39 egl-4(mg410) IV.
tm3878 is a 313 bp deletion beginning with TAGGTTTTTATCTAGGACTA and ending with ATGGATGGCCAAAGCTCGTC.
tm5814 is a 498 bp deletion beginning with GAAAAATTTCAAGTTTTAAG and ending with TGGCCAAGGTAAGATCCTCG.
Acknowledgments
We thank Dr. Hi Yo Mak for the kind gift of VS29 egl-4(mg410). Some deletion mutants were provided by the National BioResource Project (Tokyo Women’s Medical University). Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
Funding
The authors acknowledge support from NIH grant R01NS036918.
Reviewed By
AnonymousHistory
Received: May 14, 2019Accepted: May 22, 2019
Published: May 24, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Olson, AC; Koelle, MR (2019). The protein kinase G orthologs, EGL-4 and PKG-2, mediate serotonin-induced paralysis of C. elegans. microPublication Biology. 10.17912/micropub.biology.000115.Download: RIS BibTeX