Description
The daf-2 gene codes for the singular insulin-like growth factor receptor in C. elegans, acting as a control point in multiple developmental and physiological pathways that depend on the integrated response to at least forty different insulin-like peptides (Murphy and Hu, 2013). The functions of daf-2 in dauer formation, longevity, germ-line, and early larval arrest have been extensively studied. A strong daf-2 allele, e979, is homozygous viable at 15oC, despite a partially penetrant embryonic lethal phenotype at 25oC (Gems et al., 1998). The presumed null allele, m65, does not produce fertile homozygous adults due to 100% dauer arrest, and does not display embryonic lethality due to maternal rescue from heterozygous mothers. While several daf-2 alleles display low penetrance embryonic lethality at 25oC, the e979 allele displays the most penetrant embryonic arrest phenotype (Gems et al., 1998; Patel et al., 2008), making it the best choice to investigate the embryonic requirement for insulin signaling. The e979 mutation causes a C146Y substitution that is thought to interrupt an existing disulfide bond interaction with C181, destabilizing the typical fold of the insulin receptor, and impairing the L1 domain’s function in ligand binding (Patel et al., 2008).
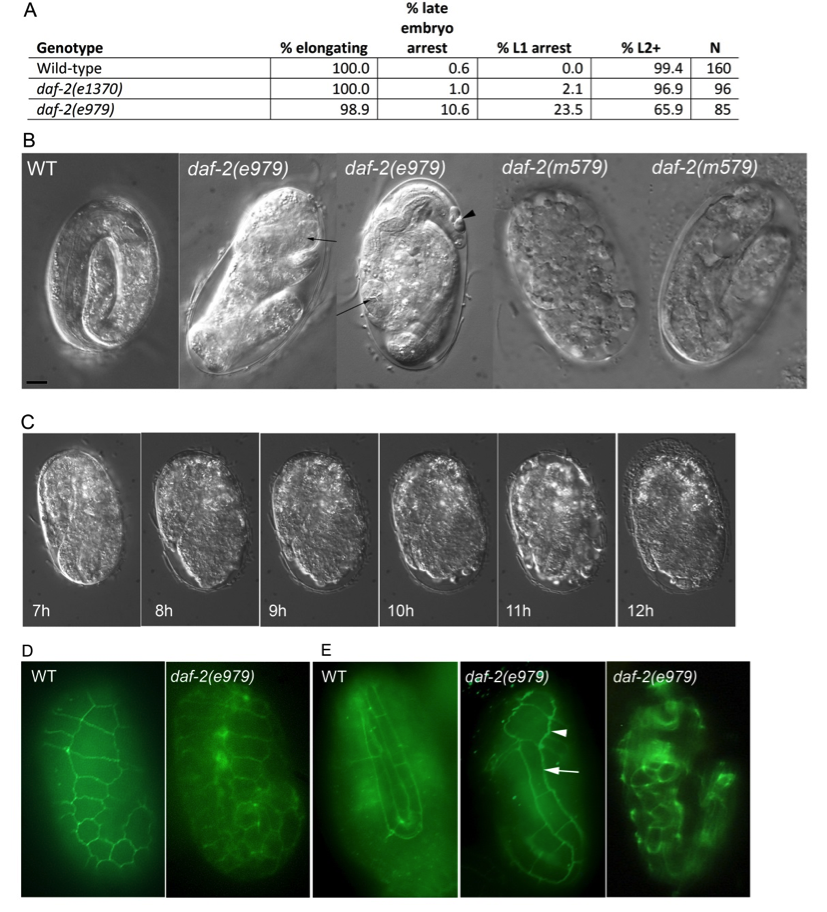
To investigate the role of insulin-like signaling in embryonic development, we examined daf-2(e979) and parallel N2 wild-type and daf-2(e1370) controls at 25oC. The canonical e1370 allele causes a strong dauer arrest phenotype, but a very low penetrance embryonic arrest phenotype. While 98.9% of daf-2(e979) embryos reached early elongation midway through embryogenesis (comma stage), 10.6% of embryos arrested without hatching (Fig. 1A). Similar to previous reports, 23.5% of daf-2(e979) animals arrested development as fully-elongated L1 animals at or after hatching (Gems et al., 1998; Baugh, 2013). Examination of arrested daf-2(e979) embryos by DIC microscopy revealed that they had some of the features of late embryogenesis, such as an apparently normal pharynx, but had failed to elongate properly (Fig. 1B). We followed five control wild-type embryos and six daf-2(e979) embryos by time-lapse microscopy over an 8 hour period at 25oC beginning at the mid-embryogenesis comma stage. While all of the wild-type and five of the daf-2(e979) embryos exhibited normal elongation and morphogenesis, one daf-2(e979) embryo failed to elongate, beginning just before the two-fold stage (Fig. 1C). The failing embryo twitched normally at this stage, indicating that it had functional muscles, but was unable to elongate past the two-fold length and retracted somewhat over a one hour period. After several hours, blebs appeared at the anterior end of the embryo and it eventually ruptured. These observations suggest that insulin-like signaling plays a role in embryonic elongation in C. elegans. A role for daf-2 in embryo elongation was previously suggested based on synthetic genetic interactions of other daf-2 alleles with the let-502 Rho-binding kinase (Piekny, et al., 2000), but has not been described for daf-2 mutations alone.
The embryonic elongation process is driven by the migration, fusion, and contraction of the hypodermal epithelium (Priess and Hirsh, 1986; Costa et al., 1998; Chisholm and Hardin, 2005). Circumferential actin microfilaments connect the longitudinal margins of the belt desmosomes surrounding each hypodermal cell and contractile activity leads to the change in hypodermal cell shape seen in elongation. Therefore, elongation depends on both the mechanistic contraction of actin microfilaments and the arrangement and differentiation of the hypodermal cells themselves.
We investigated the patterning of hypodermal cells of N2 wild type and daf-2(e979) embryos grown at 25oC prior to and during embryonic elongation using indirect immunofluorescence. The adherens junctions of the hypodermal cells were stained using an MH27 primary monoclonal antibody, which outlines the perimeters of the cells (Fig. 1DE). We examined 31 N2 wild-type and 59 daf-2(e979) embryos grown at 25oC at comma stage prior to elongation (Fig. 1D). All daf-2(e979) embryos examined showed hypodermal patterning that was similar to wild-type. Ventral views (not shown) revealed no difficulties with ventral enclosure and dorsal fusion of hypodermal cells appeared normal. We were not able to determine for certain whether anterior enclosure was normal in all embryos. Most embryos (58/59) that were undergoing elongation displayed a normal pattern of hypodermal cells. One daf-2(e979) embryo appeared to have elongated lateral seam cells over much of the body, but the H0 and H1 seam cells of the head were not elongated (Fig. 1E), suggesting that this embryo may have been in the process of failing to elongate. Later embryos that were failing to elongate showed hypodermal patterning anomalies including retracted seam cells (Fig. 1E), but it was unclear whether this was a cause or an effect of elongation failure. Taken together, these results suggest that daf-2 may play a role in the mechanistic process of elongation, but not in the initial differentiation or patterning of the hypodermal cells themselves.
We considered the possibility that the elongation defect phenotype associated with e979 could reflect a strain-specific effect due to another mutation in the DR 1942 background, despite three rounds of back-crossing. Embryonic lethality was temperature-sensitive (0/146 embryos arrested at 15oC; 0.5% reported in Gems et al., 1998), as one would expect for a defect associated with a loss of insulin-signaling. We also examined hundreds of embryos grown at 25oC in weaker daf-2(m41) and daf-2(m579) mutants to look for rare arrested embryos with phenotypes similar to daf-2(e979) embryos. The m41 and m579 mutations cause 2.7% and 4.4% embryonic arrest phenotypes, respectively (Gems et al., 1998). For m579 mutant embryos, 1/9 arrested as a morphologically-normal 4-fold embryo, 4/9 arrested as post-elongation embryos with extensive necrosis (Fig. 1B, rightmost panel), 2/9 arrested as two or three-fold embryos with apparent elongation failures (Fig. 1B, second from right panel), and 2/9 arrested as amorphous embryos for which elongation could not be judged. For m41 embryos, 10/59 arrested as post-elongation embryos with extensive necrosis, 4/59 arrested as two or three-fold embryos with apparent elongation failures, and 41/59 arrested as amorphous embryos for which elongation could not be judged. Although examination of these two weaker alleles did reveal embryos with apparent elongation defects, neither phenocopied the e979 embryonic arrest phenotype exactly. Our results are consistent with a role for daf-2 insulin signaling in the embryonic elongation process, however the phenotypic consequences of daf-2 loss-of-function appear to vary among different alleles.
Reagents
Wild-type N2, DR 1942 daf-2(e979), CB 1370 daf-2 (e1370), DR 1564 daf-2(m41), and DR1566 daf-2(m579) nematode strains were obtained from the Caenorhabditis Genetics Center. The MH27 mouse anti-AJM-1 cell junction protein monoclonal supernatant was obtained from the Developmental Studies Hybridoma Bank (AB_531819). The Alexa Fluor 488 goat anti-mouse secondary antibody was obtained from Thermo Fisher (A-11001). Indirect immunofluorescence was performed by preparing embryos from mixed cultures by bleach treatment, followed by paraformaldehyde fixation and permeabilization (Finney and Ruvkun, 1990). Both primary and secondary antibodies were diluted at 1:500. DIC images were captured from a Nikon Eclipse TE2000U inverted microscope using a 60X objective, Nikon NIS software and a PC computer. Immunofluorescence images were captured from a Nikon Optiphot UD2 microscope using a 40X objective, Nikon NIS software, and a PC computer.
Acknowledgments
Strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
Funding
This work was funded by the Muhlenberg College Biology Department and by NIGMS 1R15GM107799 grant from the NIH.
Reviewed By
AnonymousHistory
Received: May 23, 2019Accepted: January 8, 2020
Published: January 13, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Suresh, A; Wightman, B (2020). The daf-2 insulin receptor functions in C. elegans embryo elongation. microPublication Biology. 10.17912/micropub.biology.000117.Download: RIS BibTeX