Description
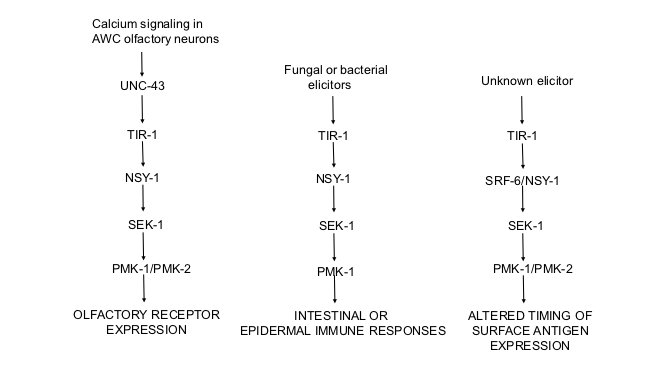
In Van Sciver et al, 2019 and Honzel et al, 2019, we showed that the gene previously described as srf-6 is actually nsy-1, which encodes NSY-1, the MAPKKK in the C. elegans p38 pathway. In earlier work, we had shown that srf-6 mutations affect timing of expression of a surface epitope (Hemmer et al., 1991). Wild-type worms express the epitope only at the L1 stage, but srf-6 mutants express it additionally at stages L2-L4 (called CLD for Constitutive Larval Display). In addition, we reported that wild-type worms can display the L1-specific epitope on stages L2-L4 when grown on a modified medium containing the concentrated extract of liquid nematode culture medium (called ILD for Inducible Larval Display, Grenache et al., 1996). Thus the expression of the L1-specific epitope appears to be controlled by an inducible switch that is under control of the srf-6 gene, which, as we now know, is a component of the p38 MAP kinase pathway in C. elegans. The srf-6 mutant phenotype, according to this model, corresponds to a switch that is constitutively “on”. srf-6(yj13) has a CLD phenotype similar to that of a large nsy-1 deletion, suggesting that SRF-6 may function to inhibit expression of the L1-specific epitope after the L1 stage.
The modulation of this switch by an extract of liquid nematode culture medium (Grenache et al., 1996) suggested to us that it might be triggered by environmental signals detected by the nematodes’ chemical senses. Genes such as daf-4 and daf-7 encode components of a TGF beta pathway that control formation of the C. elegans dauer larva in response to dauer pheromone, which is secreted by worms and detected by worm chemosensation (reviewed in Patterson and Padgett 2000). Daf-4 anddaf-7 mutants also show the CLD phenotype (Grenache et al 1996). This led us to test srf-6 mutants for chemosensory defects directly (Olsen et al 2007). We determined that srf-6(yj13) mutants are defective in chemotaxis to both water-soluble and volatile attractants. Conversely, we also tested chemosensory mutants for ILD and found that genes required for integrity of the chemosensory ciliated nerve endings are also required for ILD (Olsen et al 2007). However, genes required for olfaction were not required for ILD. We note that nsy-1 is expressed in other neurons in addition to AWC (Sagasti et al 2001), and is required in the ADF amphid neurons for pathogen-induced induction of serotonin biosynthesis (Shivers et al 2009). The tissue of expression and time of action of nsy-1/srf-6 in relation to ILD remain to be determined.
A clue as to how srf-6 might modulate surface antigen expression is found in the fact that neither srf-3(yj10) nor srf-3(yj10)srf-6(yj43) double mutants show immunofluorescence of an L1-specific epitope at any developmental stage (Hemmer et al 1991). The srf-3 gene encodes a nucleotide sugar transporter, and the pathogenic bacteria Yersinia and Microbacterium nematophilum are unable to infect srf-3 mutants (Hoflich et al 2004). Furthermore, srf-3 mutants are deficient in glycoconjugates (Cipollo et al 2004). Thus srf-6might control the expression of specific glycosylation enzymes via sensing of environmental chemical conditions.
It is well established that the outer surface of parasitic nematodes is covered in a glycoprotein surface coat. Similarities can be found between the L1-specific epitope of C. elegans and the stage-specific expression of parasitic nematode “excretory-secretory antigens”. These are also found in association with the surface of the parasite. A good example is the Toxocara canisinfective larva (L3), which has a glycoprotein coat composed primarily of an abundant mucin-like protein, TES120 (Page and Maizels 1992; Page, Rudin, and Maizels 1992). TES120 can also be found in the culture medium as a secretory product of this developmental stage (Page and Maizels 1992). The parasite has the ability to shed its TES120-containing surface coat in response to antibody binding (Smith et al 1981). We have found that mAb M37 staining is only visible on the surface of the C. elegans L1 when worms are incubated with the antibody at 0-4° C. When the sample is allowed to warm on the microscope stage, worms shed large fluorescent flakes or patches and eventually appear completely unstained (Politz and Philipp 1992). Thus a C. elegans surface epitope shows similar stage-specificity and ability to be released, as do surface coat molecules of parasitic nematodes. This raises the possibility that stage-specificity of the surface antigens of parasitic nematodes might also be controlled by a MAP kinase pathway.
Reagents
C. elegans strains used in this work were listed in Van Sciver et al., 2019, Honzel et al., 2019, and Foley et al., 2019. Strains will be sent to the CGC.
Acknowledgments
I acknowledge the many contributions of researchers in the Politz lab, published and unpublished, that contributed to the story described here.
References
Funding
I acknowledge the Office of the Dean of Arts and Sciences, Worcester Polytechnic Institute, for partial support for this project.
Reviewed By
Maria Gravato-NobreHistory
Received: June 18, 2019Accepted: June 27, 2019
Published: July 4, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Politz, SM (2019). Role of the p38 MAP kinase pathway in C. elegans surface antigen switching. microPublication Biology. 10.17912/micropub.biology.000130.Download: RIS BibTeX