Department of Chemistry and Biochemistry, Worcester Polytechnic Institute, Worcester, MA
Current Address: SBH Sciences, Natick, MA
Description
Nematodes, such as the model organism Caenorhabditis elegans, communicate environmental and developmental information with conspecifics through a class of small-molecule pheromones termed ascarosides (Butcher, 2017; Chute and Srinivasan, 2014; Ludewig and Schroeder, 2013). Nematodes share ascaroside signaling pathways (Choe et al., 2012), but are also capable of eavesdropping on chemical signals of predatory species (Liu et al., 2018). Ascarosides signal vast arrays of information, either individually or as blends, based on concentration, sex, physiological state, and other ascarosides sensed (McGrath and Ruvinsky, 2019; Pungaliya et al., 2009; Srinivasan et al., 2008; Srinivasan et al., 2012). For instance, octopamine-succinylated ascaroside #9 (osas#9) is able to signal starvation conditions in the absence of other ascarosides (Artyukhin et al., 2013).
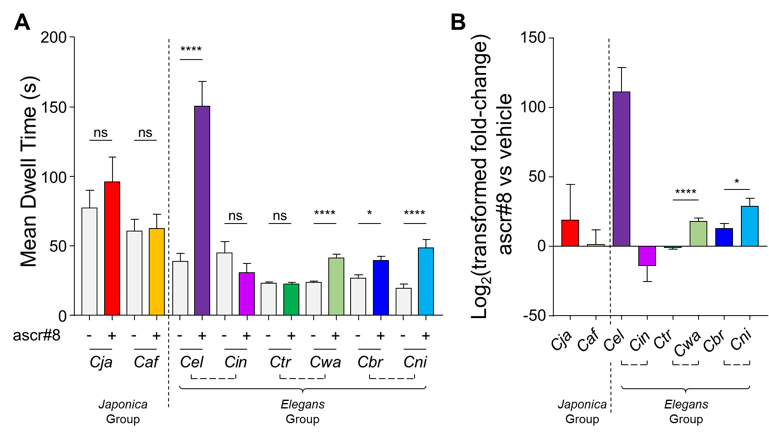
C. elegans (Cel) is an androdioecious species, with the majority of the natural population comprised of self-fertilizing hermaphrodites, and a small proportion (<0.2%) being male (Hodgkin et al., 1979). There are two other similarly androdioecious species in the genus, C. briggsae (Cbr) and C. tropicalis (Ctr). All three species evolved their hermaphroditism separately and uniquely (Ellis and Lin, 2014). Of the male-attracting ascarosides secreted by C. elegans (ascr#2, ascr#3, ascr#4, and ascr#8), ascr#8 is the most potent (Pungaliya et al., 2009). Since ascr#8 is a male attractant in this hermaphroditic species, we asked if other hermaphroditic species retained the ability to attract males using this cue. Males from the gonochoristic (male-female) sister species to C. briggsae and C. tropicalis – C. nigoni (Cni) and C. wallacei (Cwa), respectively – were also assayed for their ability to respond to ascr#8. The closest relative of C. elegans, the gonochoristic C. inopinata (Cin, formerly C. sp. 34), which has been recently characterized (Kanzaki et al., 2018), was also tested, along with the Japonica Group gonochoristic species C. japonica (Cja) and C. afra (Caf).
Dwell times were analyzed as previously described using a Spot Retention Assay (Narayan et al., 2016). Dwell times were transformed using a Base 2 Exponentiation (2n, wherein n is equal to the raw dwell time value) to generate only non-zero data in order to calculate fold-changes. The Logbase2 of the fold-changes was then calculated to normalize the data. All data sets were first checked for normality using a D’Agostino & Pearson normality test before comparisons were performed. Species in which both vehicle and ascr#8 dwell times were normally distributed were compared using a paired t-test, while those in which one or both data sets were not normally distributed were compared using a Wilcoxon matched-pairs signed rank test (GraphPad Prism 7.03). Transformed values were compared between related species via an unpaired t-test.
C. elegans responded strongly to ascr#8, supporting our previously published results using the Spot Retention Assay (Pungaliya et al., 2009; Narayan et al., 2016). The other well studied hermaphroditic model species, C. briggsae, responded significantly, although not robustly as C. elegans, spending approximately 40 seconds in ascr#8 compared to C. elegans’ dwell time of nearly 150 seconds. (Fig. 1A). Surprisingly, the sister-species of C. briggsae, C. nigoni, responded in a more robust manner (Fig. 1A, B). Similarly, while C. tropicalis exhibited no response to ascr#8 – its dwell time in ascr#8 being no different than that of the vehicle control (Fig. 1A) – C. wallacei spent significantly more time in ascr#8 than the vehicle (Fig. 1A), and therefore exhibited significantly more attraction than C. tropicalis (Fig. 1B). These data suggest that females of gonochoristic species have retained their ability to attract males via ascr#8 signaling, while in androdioecious species, this communicatory mechanism is lost, potentially due to a lack of selective evolutionary pressure. However, neither Japonica Group member responded to ascr#8, nor did the closest C. elegans relative, C. inopinata (Fig. 1A). This lack of attraction to ascr#8 may be due to the highly specialized insect-commensal life-cycle of C. inopinata. This species may have lost the necessary olfactory receptors to sense ascr#8 within its reduced genome (Kanzaki et al., 2018), in a manner similar to Trichinella (Srinivasan et al., 2013).
Given that hermaphroditism evolved multiple times during nematode evolution, it is plausible that C.briggsae and C. nigoni both inherited mechanisms to sense ascr#8. However, given the recent evolution of C. briggsae (between 100,000 to 1 million years ago) (Cutter et al., 2010; Thomas et al., 2015), the reduced response compared to C. nigoni indicates less selective pressure to retain ascr#8 sensing mechanisms. Similarly, C. tropicalis likely evolved the ability to self-fertilize even before the nigoni-briggsae split (Stevens et al., 2019), leading to its inability to respond attractively to ascr#8. Together, these data suggest that ascr#8 functions as a C. elegans species-specific male attractant, with other species having lost the necessary mechanisms to sense and respond to this chemical. As different neurons and receptors may play roles in the ascr#8-mediated dauer development pathways, the comprehensive ability to sense this pheromone in the species tested is yet to be determined.
Reagents
The C. elegans strain CB1489 (him-8(e1489)) was obtained from Maureen Barr at Rutgers University. RE980 (C. briggsae him-8(v188)) and RE1017 (C. tropicalis (him-8(v287)) were generously provided by Ronald Ellis at Rowan University. The C. wallacei (JU1873), C. nigoni (JU1422), C. afra (JU1286), and C. japonica (DR5081) wild isolates were obtained from the Caenorhabditis Genetics Center. C. inopinata (NK74SC) was generously provided by the Forestry and Forest Products Research Institute in Japan.
Acknowledgments
We would like the thank Dr. Erich Schwarz (Cornell University) for valuable insights in interpreting our results. We would like to thank the Forestry and Forest Products Research Institute in Japan for providing the C. inopinata strain even before publication. We also like to thank Dr. Ronald Ellis (Rowan University) for protocols regarding maintenance of C. briggsae him-8 and C. tropicalis him-8 strains.
References
Funding
This work was supported by a grant from the NIH (R01DC016058 to J.S.).
Reviewed By
Katja R KasimatisHistory
Received: June 20, 2019Accepted: July 8, 2019
Published: July 19, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Reilly, DK; Randle, LJ; Srinivasan, J (2019). Evolution of hermaphroditism decreases efficacy of Ascaroside#8-mediated mate attraction in Caenorhabditis nematodes. microPublication Biology. 10.17912/micropub.biology.000134.Download: RIS BibTeX