Description
Genomic editing of the roundworm Caenorhabditis elegans using the CRISPR/Cas9 system has allowed for widespread creation of null mutants – vital for scientific understanding of this model organism. A closely-related nematode species, Caenorhabditis briggsae, is emerging as an alternative model organism to better understand how findings in C. elegans can broaden and develop the larger field of nematology (Gupta et al. 2007). To that end, we have developed an effective and efficient co-conversion CRISPR/Cas9 system for use in C. briggsae using the gene dpy-10.
We modified the universal STOP-IN cassette method, as described by Wang et al. (2018), for use in C. briggsae (Wang et al. 2018). Using the wildtype AF16 strain, we tested the method by choosing the gene Cbr-dpy-10 because of its readily observed predicted phenotype. Cbr-dpy-10 is a predicted one-to-one ortholog of C. elegans dpy-10, which encodes a protein important for cuticle development and has a phenotype characterized by short, fat animals relative to wild type (Brenner 1974).
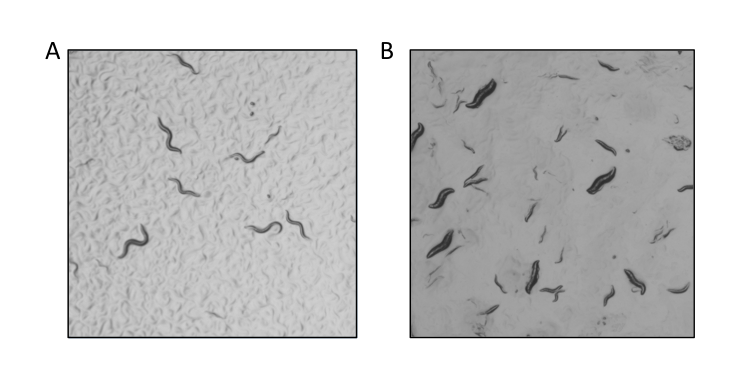
A universal STOP-IN allele of Cbr-dpy-10, sy1387, was generated and confirmed by genotype sequencing. The expected phenotype was subsequently observed, consistent with the creation of a null allele (Figure 1). Surprisingly, sy1387 is dominant. Injections of 20 animals produced 15 successful injections; 247 animals from these 15 injections (F1) were singled out and allowed to self-fertilize to produce F2. 81% of the F1 Dumpy progeny were homozygous as evidenced by their segregation of only Dumpy progeny – one of these candidates became sy1387. 2% of the F1s did not produce progeny. 17% of the 247 F1 Dumpy progeny were heterozygotes and had a mixture of Dumpy and non-Dumpy progeny; these non-Dumpy F2s only produced non-Dumpy progeny. This discovery of a dominant mutation will allow for more effective use of this as a co-conversion marker when screening for other mutations. We used Cbr-dpy-10 as a potential co-CRISPR marker for a second target that will be described elsewhere. We used PCR to detect insertion of a STOP-IN cassette at this other locus; we screened 39 Dumpy strains to obtain 11 candidates for our target gene, from which we have three STOP-IN alleles.
Methods
Request a detailed protocolWe used the universal STOP-IN cassette method essentially as described in Wang et al. (2018). Potential guide sequences were followed by a 5’-NGG-3’ PAM site and were close to the start codon ATG of the target gene. The guide sequence for Cbr-dpy-10 used in this protocol was ATTCGCGTCAGATGATGTAC, located at the beginning of the gene’s second exon. To detect the stop-in insertion into Cbr-dpy-10, we used forward primer GAAAAACAACGGCAGAGACG and reverse primer TCCGCTTCCATAAGCACCAC.
In sy1387, the second exon of Cbr-dpy-10 (shown below) was changed with the 41 basepair insert highlighted in red. This caused the early introduction of a stop codon (underlined) and a subsequent frameshift.
CTATCGAACATTCTCGCTGACAACGAACTATTCGCGTCAGATGATGGAAGTTTGTCCAGAGCAGAGGTGACT AAGTGATAAGCTAGCTACCGGTGTGTCACAGGTCTTCAAATCGGATTTAGTTTGTTCTCATTCGTTATCGTT TGTGCCCTTCCTATTATGTACAATCATGTCCAGAACACAATTACTTATGTTGACAGAGAAATAGTGGCTTAT TGCGAA
Reagents
All CRISPR/Cas9 system reagents were ordered from IDT except for the Cas9 protein, which was kindly provided by Tsui-Fen Chou. Sequences were downloaded from WormBase.
Strain Generated:
PS8520 Cbr-dpy-10 (sy1387) II
Acknowledgments
We thank Heenam Park for her discussion and comments.
References
Funding
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE 1745301 and the NIH under Grant No. R24 0D023041.
Reviewed By
AnonymousHistory
Received: September 12, 2019Accepted: September 30, 2019
Published: October 11, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Cohen, SM; Sternberg, PW (2019). Genome editing of Caenorhabditis briggsae using CRISPR/Cas9 co-conversion marker dpy-10. microPublication Biology. 10.17912/micropub.biology.000171.Download: RIS BibTeX