Institute of the Environment and Sustainability, University of California, Los Angeles (UCLA), Los Angeles, CA 90095
Description
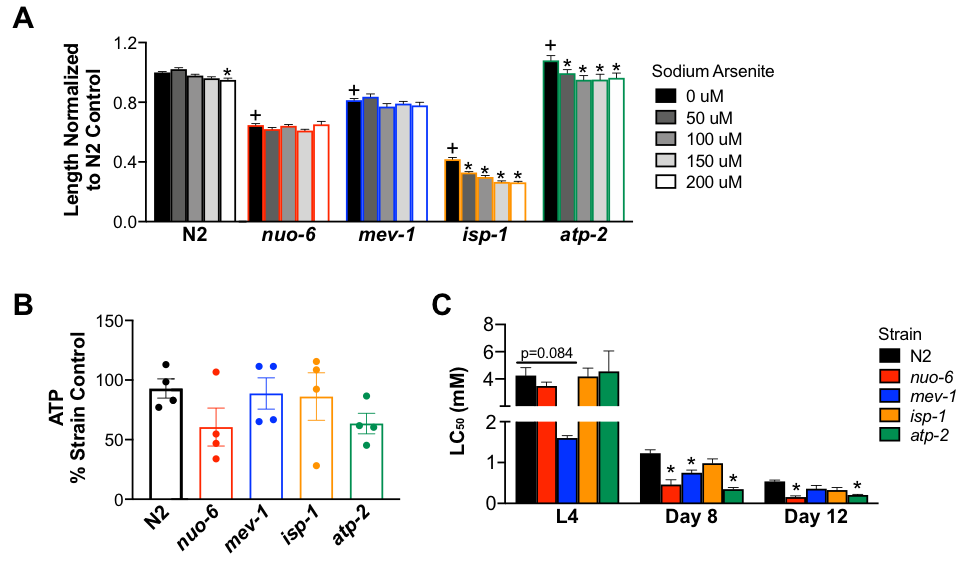
Arsenic is a toxicant with multiple intracellular targets, including many mitochondrial enzymes. Previous work in C. elegans has shown that strains deficient in complexes I, II, and III (but not complex V) have increased sensitivity to sodium arsenite, suggesting that the electron transport chain is a key target of arsenic toxicity (Luz et al. 2016). However, in that work, the only outcome used to determine strain sensitivity to arsenic was larval growth. Based on these results, we used complex mutants (nuo-6 (complex I), mev-1 (complex II), isp-1 (complex III), and atp-2 (complex V)) to further explore the physiological effects of sodium arsenite induced electron transport chain dysfunction. We used three different assays to ask the same question: which electron transport chain mutant strain is most sensitive to sodium arsenite? Interestingly, we found that strain sensitivity to sodium arsenite depends on the outcome measured. These data indicate that single assays may not provide a complete picture of nematode response to an exposure and suggests that complete characterization of nematode response requires analyzing multiple endpoints.
Nematodes were synchronized as L1 larvae by standard egg prep methods. For all experiments, biological replicates were performed with independent egg preps on different days. To minimize the potential for different bleaching events to affect outcomes and confound the strain-specific differences we sought to characterize, strains compared in a given experiment were bleached at the same time, or mutant strain data was normalized to N2s bleached at the same time (exceptions to this statement are the lethality curves generated for nuo-6 and isp-1 mutants at the L4 stage which were performed in isolation on separate days) (Lewis and Felming 1995). First, synchronized nematodes were treated with sodium arsenite in liquid K-medium at concentrations from 0-200 μM for 72 hours at 20 °C. Worm length was measured following this exposure using the NIS-Elements BR 3.2 program (Bodhicharla et al. 2014) (Fig. 1A). The concentrations of sodium arsenite used in this assay were lower than those previously reported (Luz et al. 2016) and yielded slightly different results. We found that isp-1 and atp-2 strains had decreased worm length when exposed to low concentrations of sodium arsenite, but that length of the nuo-6 and mev-1 strains were not affected. Next, we measured ATP in worms following sodium arsenite exposure (Fig. 1B). Synchronized worms were exposed to 500 μM sodium arsenite beginning 2 days post hatch for 48 hours. Immediately after the exposure, steady state ATP levels were measured using the Promega CellTiterGlo Luminescent assay and normalized to protein per worm (adapted from Bailey et al. 2016). Although we report no significant differences, there is a strong trend of decreased ATP in the nuo-6 and atp-2 mutant strains. Finally, the 24 hour LC50 to sodium arsenite was determined in the mutant strains at different life stages. Strains were exposed to various concentrations of sodium arsenite for 24 hours at the L4 stage, 8 days of age, and 12 days of age. 10 worms per well (three wells per concentration) were exposed and wells were scored immediately after the exposure to determine percent alive in each well. At the L4 stage, only the mev-1 mutant strain appeared to have altered sensitivity to sodium arsenite. However, in the 8-day old worms, the nuo-6, mev-1, and atp-2 strains were more sensitive to sodium arsenite compared to the N2 strain. This was also observed in the 12-day old worms with the exception that the mev-1 strain did not show increased sensitivity to arsenic.
Together, our data shows that strain sensitivity to arsenic is dependent on the outcome measured. For example, the isp-1 strain has decreased growth when exposed to low concentrations of sodium arsenite but does not appear to have differences in ATP content or lethality compared to N2 controls. In contrast, the nuo-6 strain showed no difference in growth compared to N2 controls during sodium arsenite exposure but appeared to have decreased ATP and had a significantly decreased LC50 at 8 and 12 days of life. Additionally, we show that even within the same assay, strains responded differently to sodium arsenite exposure at different life stages. While mev-1 appeared to be most sensitive to sodium arsenite at the L4 stage, the mev-1 strain did not show an increased sensitivity to sodium arsenite in the 12-day old worms. Three separate assays with different exposure concentrations, age of worm, and outcome measured yielded different results to our original research question. This result highlights the importance in toxicology of not just genetic background in modulating sensitivity, but also age—i.e., gene × environment × age interactions. The mechanisms responsible for these differences remain to be determined but may include toxicokinetic (e.g., cuticular and xenobiotic defense mechanisms) as well as toxicodynamic (e.g., differential reliance of specific mitochondrial metabolic processes at different life stages) differences. This work reveals how assay design influences answers to scientific questions, and highlights the importance of measuring multiple outcomes to characterize nematode strains.
Reagents
Strains
Wild type (N2 Bristol)
ETC Complex I: nuo-6(qm200), CGC Strain MQ1333
ETC Complex II: mev-1(kn1), CGC Strain TK22
ETC Complex III: isp-1(qm150), CGC Strain MQ887
ETC Complex V: atp-2(ua2), CGC Strain LB127
Acknowledgments
We thank Anthony Luz for adaptation of the CellTiterGlo Luminescent assay for ATP measurements. We thank Ian Ryde for assistance with experimental procedures.
References
Funding
NIH P42ES010356
Reviewed By
Erik AndersenHistory
Received: October 11, 2019Accepted: October 17, 2019
Published: October 22, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Hershberger, KA; Leuthner, TC; Waters, TA; Meyer, JN (2019). Caenorhabditis elegans strain sensitivity to sodium arsenite exposure is varied based on age and outcome measured. microPublication Biology. 10.17912/micropub.biology.000186.Download: RIS BibTeX