Department of Medicine, Division of Cardiology, McGill University and the Cardiovascular Health Across the Lifespan Program, Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada
Department of Medicine, Division of Endocrinology and Metabolism, McGill University and the Metabolic Disorders and Complications Program, Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada
Description
The excessive storage of neutral lipids in lipid droplets (LDs) is a consequence of excess dietary nutrient uptake and the primary cause of major metabolic disorders, including obesity, diabetes, and atherosclerosis (Eckel et al., 2005). Several studies investigating proteins associated with the monolayer surface of LDs have identified the Rab18 GTPase as a component with a role in the regulation of fat storage and lipid mobilization (Martin et al., 2005; Ozeki et al., 2005). Rab18, a small GTPase protein localized on the surface of LDs, plays a key role in several LD-related processes, including lipogenesis, lipolysis, and lipophagy (Pulido et al., 2011; Schulze et al., 2017). Although Rab18 has been previously associated with multiple roles in lipid metabolism, including mediating the apposition of LDs to the ER, its fundamental function remains disputed (Ozeki et al., 2005). To better understand the function of Rab18 in fat control at the organismal level, we proceeded to investigate the effect of rab-18 knockout on overall lipid abundance in C. elegans.
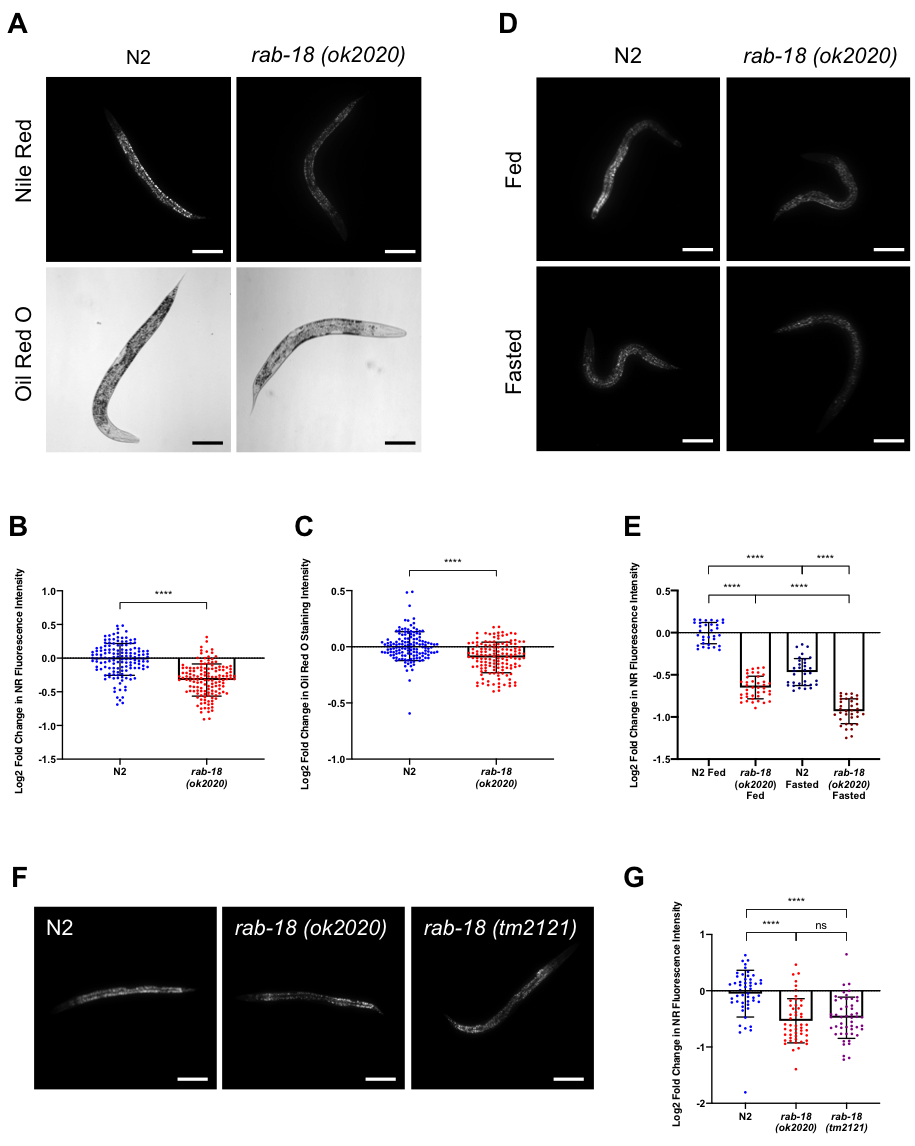
To determine if RAB-18 regulates fat storage in C. elegans, we first investigated the fat content of rab-18(ok2020) mutants compared to wild type (N2) worms by performing fixed Nile Red (NR) and Oil Red O (ORO) staining experiments under fed conditions. Each NR and ORO experiment was performed with 5 different samples of N2 and rab-18(ok2020) worms and 10 worms were analyzed from each sample. Three separate NR and ORO experiments were performed for a total of 150 images per strain. rab-18 mutants displayed a significant reduction in NR fluorescence intensity (Figure 1A, B) and ORO staining intensity (Figure 1A, C) compared to the control N2 worms. This reduction suggests a significant decrease in lipid content in rab-18(ok2020) mutants compared to N2 worms. The less pronounced reduction in the fat content of rab-18(ok2020) mutants observed in ORO experiments is attributed to the lower sensitivity of ORO in quantifying changes of lipid abundance compared to NR (Escorcia et al., 2018).
The fixed NR staining experiment of wild type (N2) and rab-18(ok2020) mutant worms was also performed under short-term (6 hours) fasting conditions to determine whether the previously observed decrease in fat content of mutant worms under fed conditions was amplified or reduced under short-term fasting conditions. Moreover, fixed NR staining under fasting conditions serves as an internal control to ensure the accuracy of the staining method as the reduction in LD fat content through lipolysis upon short-term fasting is widely acknowledged in the literature (Srinivasan et al., 2015). Three separate NR staining experiments were performed and 10-15 images were captured per strain for each experiment. Representative images are shown in Figure 1D and quantified in Figure 1E. A significant reduction in NR fluorescence intensity was observed in rab-18(ok2020) mutants as compared to wild type (N2) under fasted conditions, suggesting a significant decrease in lipid content. Moreover, we demonstrate a decrease in fat content under short-term (6 hours) starving conditions in both wild type and rab-18(ok2020) mutant worms, which is in line with findings that short-term fasting depletes body fat stores due to the increased expression of ATGL (atgl-1) lipase mediating lipolysis (Srinivasan et al., 2015). Thus, RAB-18 promotes lipid storage under both fed and fasted conditions and is not required for lipolysis during short-term fasting.
To rule out the possibility that background mutations might have affected our results, we performed NR staining experiments using an additional rab-18 deletion allele, tm2121. Five separate NR staining experiments were performed on wild type (N2), rab-18(ok2020), and rab-18(tm2121) mutant worms and 10 images were captured per strain in each experiment, with representative images shown in Figure 1F and quantified in Figure 1G. Like the rab-18(ok2020) mutant, rab-18(tm2121) worms displayed a significant reduction in lipid staining compared to N2 worms. There was no statistically significant difference between the reduction in fat content observed in rab-18(ok2020) and rab-18(tm2121), which reinforces the notion that the observed phenotype is caused by the deletion of rab-18.
To confirm that changes in fat storage are due to loss of rab-18 rather than differences in animal health, we tested for differences in fertility, viability and growth. Ten animals per strain were permitted to lay eggs for 8 hours and the number of eggs laid was quantitated as a measure of fertility. Three days later, the number of animals were counted as a measure of viability, and the number of animals that reached maturity (fertile adults) as a measure of growth. Relative to wild type N2 (n=305), the number of eggs laid by rab-18(ok2020) and rab-18(tm2121) was 90% (n=273) and 79% (n=240), viability was 99% and 93% and the number of animals reaching maturity was 86% and 63%, respectively. Thus, rab-18(ok2020) grew significantly slower than N2 (P<0.0001), while rab-18(tm2121) was significantly less fertile (P<0.01), viable (P<0.001) and grew more slowly (P<0.0001) than N2. Notably, rab-18(ok2020) and rab-18(tm2121) have significant differences in growth (P<0.0001) and viability (P<0.05) that could be due to background mutations in these two independently derived strains. Since rab-18(ok2020) and rab-18(tm2121) have the same level of reduced fat staining as compared to wild type (N2),we attribute this to the loss of rab-18 gene function rather than a difference in health. Our findings demonstrate a role for the RAB-18 GTPase in regulating fat content under both fed and starved conditions, and establish C. elegans as a model for analysis of RAB-18 in the regulation of lipid storage.
Methods
Request a detailed protocolC. elegans worms were maintained at 20°C on nematode growth medium (NGM) seeded with HB101 E. coli. For fasting experiments, fed L4 stage worms were transferred to unseeded NGM plates for 6 hours. L4 stage worms were fixed in isopropanol and stained with NR (Invitrogen) or ORO (Sigma-Aldrich) as previously described (Escorcia et al., 2018). Slides carrying the NR stained worms were visualized under the FITC/GFP channel of an Axio Zeiss A1 compound microscope (Zeiss, Oberkochen, Germany) with a 10X objective. Images were captured using an Axio Cam Mrm camera and Axiovision software (Zeiss) with an exposure of 200ms, and maximal UV strength. Slides with ORO stained worms were visualized by bright-field microscopy with an exposure of 1ms. An ImageJ macro was used to partially automate as well as to increase the efficiency and accuracy of the quantification of the NR/ORO stained images.
Image Quantification:
- Upload micrographs to ImageJ. In the Image pull down-menu, under the Type function, convert the image into an 8-bit format.
- In the Image pull-down menu, under the Adjust function, use Auto Local Adjust (method=mean, radius=100, select “white objects on black background”) to create a threshold for your mask.
- In the Process pull-down menu, select the Filters function and use Minimum (radius=1) to create a filter that eliminates signals below a specific minimum size.
- Run the ROI Manager to create a mask for each worm to be quantified. Reset the ROI Manager prior to quantifying each worm.
- Under the Analyze pull-down menu, select the Analyze Particles function (size=300-infinity, pixel circularity-0.0-1.00, show=masks, select “add to manager”) and select the image for intensity quantification to measure the relative NR fluorescence or ORO staining of each worm.
Computing of the Log2-fold change in NR/ORO staining intensities of rab-18 mutants compared to N2 worms was achieved using the equation: log2 (staining intensity of rab-18 mutant worm / average staining intensity of N2).
Statistics
Statistical analysis was done using Graphpad Prism software. Student’s t-test and with an analysis of variance (ANOVA) was used for fat staining, unpaired t-test for fertility and a Fisher’s exact test for viability and growth.
Reagents
| Strain Name | Genotype | Source |
| N2 | wild type | Caenorhabditis Genetics Center, University of Minnesota |
| RB1638 | rab-18(ok2020) III | Caenorhabditis Genetics Center, University of Minnesota |
| FX2121 | rab-18(tm2121) III | Shohei Mitani, National BioResource Project, Japan |
Acknowledgments
We thank Amr Omer for his invaluable help in partially automating the quantization of the staining images, Sarah Gagnon for comments on the manuscript, Içten Meras for help with NR staining and Shohei Mitani for the FX2121 strain.
References
Funding
Z.R. was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Undergraduate Student Research Award (USRA). This work was supported by a Canadian Institute of Health Research (CIHR) project grant (PJT-159725) to C.E.R. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Reviewed By
AnonymousHistory
Received: October 17, 2019Accepted: December 20, 2019
Published: December 27, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Ratemi, Z; Kiss, RS; Rocheleau, CE (2019). C. elegans rab-18 mutants display reduced lipid content under fed and fasted conditions. microPublication Biology. 10.17912/micropub.biology.000188.Download: RIS BibTeX