Description
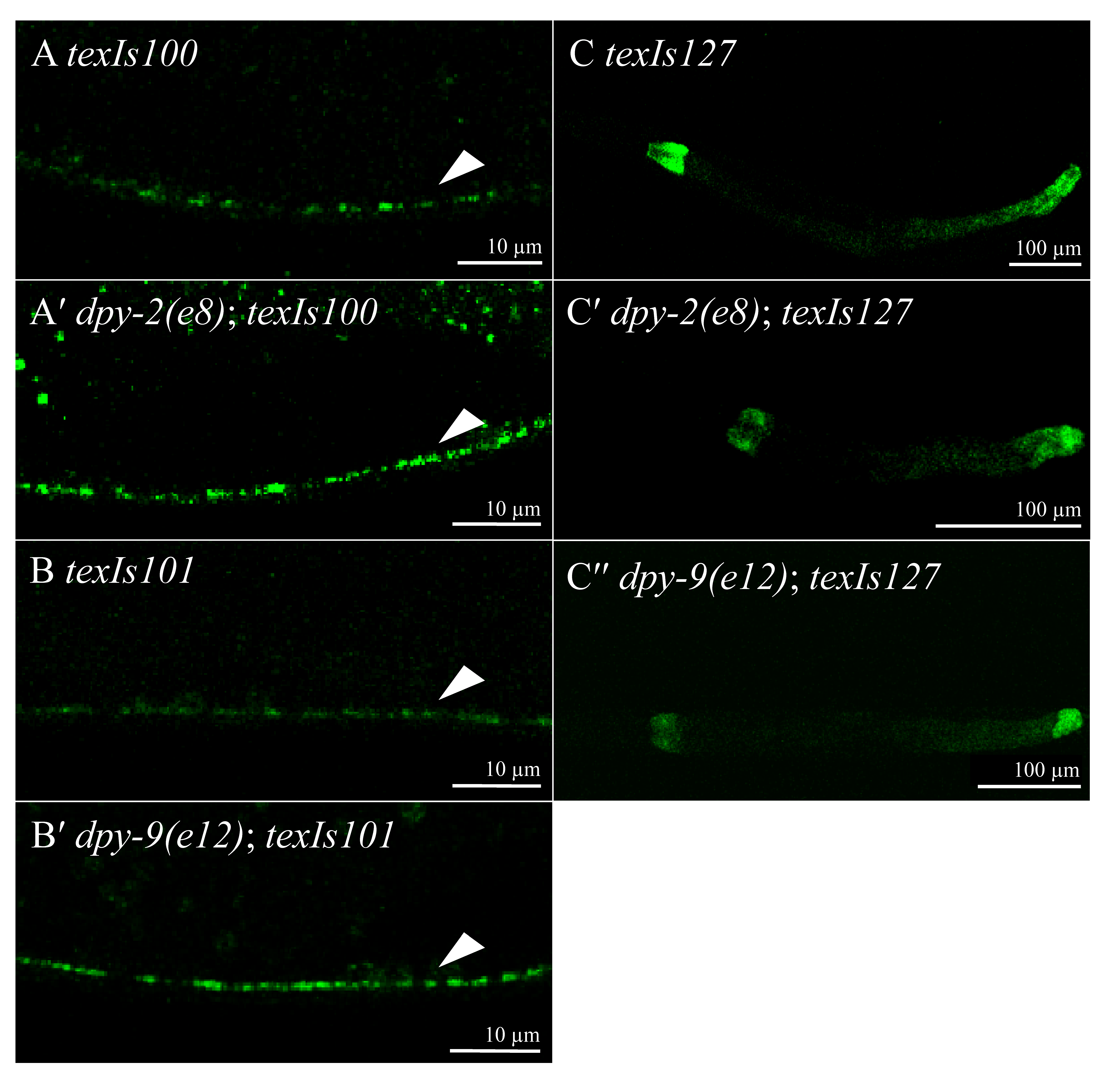
Loss of some cuticle collagens negatively affects DBL-1 pathway signaling in a stage-dependent manner (Lakdawala et al. 2019; Madaan et al. 2019). We previously observed that in one-day old adult animals, loss of dpy-2 or dpy-9 had no effect on GFP::DBL-1 expressed from the dbl-1 promoter (Beifuss and Gumienny 2012; Lakdawala et al. 2019). We also observed that expression of spp-9p::gfp, a reporter that is negatively regulated by the DBL-1 pathway, was not affected in one-day old adult animals (Roberts et al. 2010; Lakdawala et al. 2019). Post-embryonic expression of dpy-2 and dpy-9 is highest in L2 and L3, but low in L4 and even lower in young adults (Gerstein et al. 2010). Because cuticle secreted in one stage creates the cuticle in the next stage, this is consistent with the observation that loss of dpy-2 and dpy-9 has no effect on DBL-1 signaling in the adult (Hall and Altun 2008; Lakdawala et al. 2019). However, the DPY-2 and DPY-9 expression patterns led us to ask if DBL-1 signaling is affected at L4 by loss of dpy-2 or dpy-9. To our surprise, we found that dpy-2(e8) or dpy-9(e12) resulted in significant increases of GFP::DBL-1 fluorescence within DBL-1-secreting cells in L4 animals compared to control populations (Figure 1, Table 1). We also tested DBL-1 pathway reporter activity in these dpy-2 and dpy-9 mutants. Consistent with the increased GFP::DBL-1 fluorescence at L4, we observed significantly decreased fluorescence from the spp-9p::gfp reporter at L4 (Figure 1, Table 1). These results are consistent with DPY-2 and DPY-9 affecting DBL-1 signaling at the L4 stage but not at the adult stage. This suggests that these two collagens have a stage-specific effect on DBL-1 signaling, but this effect is normally inhibitory, as loss of dpy-2 or dpy-9 increased GFP::DBL-1 fluorescence and decreased spp-9p::GFP fluorescence.
| Table 1: Effects of dpy-2 and dpy-9 gene mutations on GFP::DBL-1 and DBL-1 pathway reporter spp-9p::GFP fluorescence | ||||||
| Gene | Genotype | GFP::DBL-1 fluorescence % control ± 95% CI |
P value | Genotype | spp-9p::GFP % control ± 95% CI |
P value |
| Animals at L4 stage | ||||||
| control | texIs100 | 100±29.58 | – | texIs127 | 100±7.94 | – |
| control | texIs101 | 100±54.28 | – | – | – | – |
| dpy-2 | dpy-2; texIs100 | 155.47±55.58 | 0.0263 | dpy-2; texIs127 | 80.26±10.62 | 0.0009 |
| dpy-9 | dpy-9; texIs101 | 212.94±98.06 | 0.0009 | dpy-9; texIs127 | 84.37±9.56 | 0.0028 |
| Animals at adult stage (data from (Lakdawala et al. 2019)) | ||||||
| control | texIs100 | 100±15.57 | – | texIs127 | 100±11.47 | – |
| control | texIs101 | 100±25.95 | – | – | – | – |
| dpy-2 | dpy-2; texIs100 | 115±52.15 | 0.5080 | dpy-2; texIs127 | 107.04±12.20 | 0.2344 |
| dpy-9 | dpy-9; texIs101 | 95.02±29.01 | 0.7248 | dpy-9; texIs127 | 100.29±10.24 | 0.9533 |
Methods
Request a detailed protocolNematode maintenance and imaging All the strains were maintained at 20°C on EZ media (Madhu et al. 2019). L4 animals were anesthetized using 1 mM levamisole hydrochloride (Sigma, St. Louis, MO) and imaged on a Nikon A1 confocal system (Nikon Instruments, Melville, NY). GFP::DBL-1 fluorescence was captured using a 60X objective and spp-9p::gfp fluorescence was captured using a 10X objective. The imaging conditions were optimized and kept constant between control and experimental samples. Nikon NIS Elements AR-5.02 software was used to quantify fluorescence intensities. Statistical analyses were performed using the unpaired t-test to compare control and experimental sample means. ‘‘% control ± 95% CI” is the ratio of the indicated strain mean to the control strain mean ± 95% confidence interval. n=10 for each strain imaged for the GFP::DBL-1 experiment, and n=15 for each strain imaged for the spp-9p::GFP experiment.
Reagents
Strains
Strains used in this study are:
TLG182 texIs100 [dbl-1::dbl-1:gfp; ttx-3p::rfp] IV
TLG205 texIs101 [dbl-1::dbl-1:gfp; ttx-3p::rfp] V
TLG697 texIs127 [spp-9p::gfp] X
TLG701 dpy-2(e8); texIs100
TLG702 dpy-9(e12); texIs101
TLG725 dpy-2(e8); texIs127
TLG724 dpy-9(e12); texIs127
Strains are available upon request.
Acknowledgments
We thank Cathy Savage-Dunn for helpful comments. We thank all our lab members for useful discussions. Some strains were obtained from the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We thank WormBase.
References
Funding
This work was supported by NIH grants R01 GM097591, a TWU Chancellor’s Research Fellowship to TLG, and internal funding by Texas Woman’s University.
Reviewed By
AnonymousHistory
Received: November 15, 2019Accepted: November 21, 2019
Published: December 9, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Lakdawala, MF; Gumienny, TL (2019). Loss of dpy-2 and dpy-9 has stage-specific effects on DBL-1 pathway signaling. microPublication Biology. 10.17912/micropub.biology.000191.Download: RIS BibTeX