Description
Leucine-rich repeat (LRR) domain-containing proteins play central roles in organizing neural connectivity. The LRR is a protein-recognition motif and proteins with extracellular LRR (eLRR) domains mediate intercellular communication and cell adhesion, which in turn regulate neuronal processes such as axon guidance, target selection, synapse formation and stabilization of connections (de Wit et al. 2011). The LRR-domain containing Slits and their Robo receptors are one of the best characterized examples of ligand-receptor pairs that regulate midline crossing and axon guidance in both Drosophila and vertebrates (Brose et al. 1999; Dickson and Gilestro 2006). There are 66 eLRR proteins in Drosophila, many of which are expressed in the nervous system and exhibit strikingly specific expression patterns, often labeling distinct subpopulations of neurons (Lauren et al. 2003; Dolan et al. 2007). The binding partners and functions of many of these eLRR proteins remain unknown.
We have previously described a novel method to identify ligands and/or binding partners for extracellular proteins (Fox and Zinn 2005; Lee et al. 2013; Ozkan et al. 2013). This method involves using fusion proteins containing the extracellular domain (ECD) of a protein fused to a pentamerization domain (COMP), followed by human placental alkaline phosphatase (AP). These AP fusion proteins are used to stain live-dissected stage 16 Drosophila embryos. The resulting staining patterns can be used as a template to identify expression patterns of the binding partners of the AP fusion protein. Using this technique, we have identified ligands for the receptor tyrosine phosphatases Ptp10D, Lar and Ptp69D (Bali et al. 2019; Fox and Zinn 2005; Lee et al. 2013). Here, we describe novel binding patterns for 5 eLRR proteins using their respective AP fusion proteins.
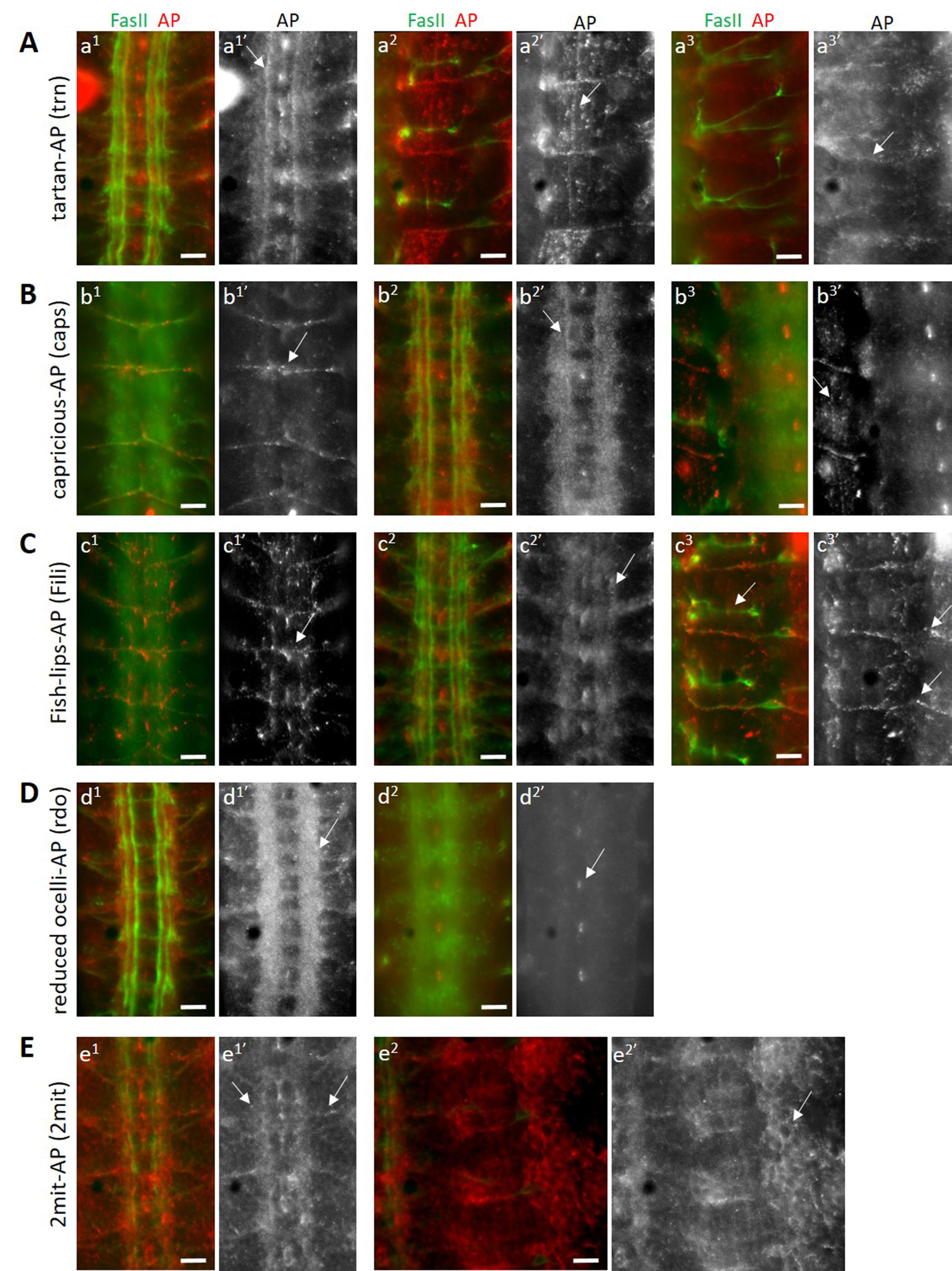
Tartan (trn) and Capricious (caps) are two closely-related eLRR proteins with known functions in embryonic motor axon guidance and the innervation of antennal lobe glomeruli by olfactory sensory axons (Kurusu et al. 2008; Hong et al. 2009). Studies of trn and caps single and double mutants suggest that the two genetically interact and may function through a common binding partner (Milan et al. 2005; Kurusu et al. 2008). Tartan may be a substrate for the receptor tyrosine phosphatase Ptp52F (Bugga et al. 2009). We stained wild-type live-dissected stage 16 Drosophila embryos with trn-AP and caps-AP fusion proteins separately, and found distinct as well as overlapping staining patterns for both fusion proteins. Both trn-AP and caps-AP bind to longitudinal axons in the ventral nerve cord (VNC), with stronger binding seen in one particular axon bundle close to the midline (arrows, a1’ and b2’). Both also show binding to muscles (arrows, a2’ and b3’), indicating that they interact with a binding partner expressed on the surface of muscles. trn-AP shows binding to a subset of sensory neurons (arrow, a3’), which caps-AP does not. In addition, caps-AP binds to the transverse nerve, which emanates from the midline and is located on the dorsal side of the VNC (arrow, b1’).
Fish-lips (Fili) is an eLRR with roles in the regulation of apoptosis (Adachi-Yamada et al. 2005) and olfactory receptor neuron (ORN) targeting in the antennal lobe (Xie et al. 2019). It is expressed at moderately-high levels during embryonic stages 12 – 17 and during 24 – 48 hours after puparium formation (modENCODE Temporal Expression Data, FlyBase). These developmental stages correspond to peak synaptogenesis times, implying a developmental role of Fili in regulating synaptogenesis. Thus, identification of binding partners of Fili is crucial to understand its roles in CNS development. Staining of wild-type stage 16 embryos with Fili-AP fusion protein shows a restricted binding pattern in the CNS, indicating a similar restricted expression pattern of its binding partners. It binds to a set of dorsal midline neurons (arrow, c1’) and a subset of longitudinal axons in the VNC (arrow, c2’). A subset of midline cells, putatively glial cells are also labeled with Fili-AP. Strong binding is seen to the transverse nerve in the VNC (c1) and in the periphery (arrow, c3’), while no labeling is seen to the SNa in the same focal plane (arrow, c3).
Reduced ocelli (rdo) is a gene that regulates ocelli development (Caldwell et al. 2007) and encodes an eLRR protein of unknown function. Caldwell et al. 2007 showed a broad expression pattern of the encoded protein in the adult nervous system. We performed staining of wild-type stage 16 embryos with rdo-AP fusion protein and found a very strong binding signal in the longitudinal and commissural axons of the VNC (arrow, d1’). This binding was limited to the VNC, and no binding was observed to the muscles (data not shown), indicating that the eLRR encoded by rdo interacts with neuronal-specific ligands. We also observed binding in a subset of midline glial cells in the VNC (arrow, d2’).
2mit is another gene encoding a putative eLRR and is expressed in the developing nervous system. It has a putative role in regulating short-term memory (Baggio et al. 2013). No other information is known about this eLRR. We stained wild-type stage 16 embryos with 2mit-AP fusion protein and saw a wide pattern of binding by this fusion protein, unlike the other restricted patterns observed above. Both longitudinal, commissural as well as exiting motor axons in the VNC are labeled by 2mit-AP (arrows, e1’). Moreover, we observed a pan-cellular pattern of labeling in the periphery as well as in the VNC, where 2-mit-AP binding signal is seen on the surface of cells, resulting in a cell-membrane staining pattern (arrow, e2’). This implies that the eLRR encoded by 2mit is capable of interacting with ligands expressed on neuronal as well as non-neuronal cell types.
These binding patterns provide clues to the expression patterns of proteins that these eLRRs might interact with to regulate various developmental processes.
Reagents
y1 w1 (FlyBase ID FBst0001495)
References
Funding
This work was supported by National Institutes of Health grant R37 NS28182 to K.Z. and by Gordon Ross Postdoctoral Fellowship to N.B.
Reviewed By
Liqun Luo and Steven MarygoldHistory
Received: December 6, 2019Accepted: December 16, 2019
Published: December 18, 2019
Copyright
© 2019 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Bali, N; Zinn, K (2019). Visualization of binding patterns for five Leucine-rich repeat proteins in the Drosophila embryo. microPublication Biology. 10.17912/micropub.biology.000199.Download: RIS BibTeX