University of Puerto Rico-Río Piedras, Río Piedras, PR
Description
Mature oocytes arrest in meiosis by the end of oogenesis and need to be activated in order to proceed to embryonic development. This egg activation process encompasses a series of events that transition the oocyte to a developing embryo, including meiosis resumption, maternal protein translation/modification, maternal mRNA processing, and cytoskeleton and eggshell changes (reviewed in Avilés-Pagán and Orr-Weaver, 2018; Horner and Wolfner, 2008a; Kashir et al., 2014; Krauchunas and Wolfner, 2013). The triggers of egg activation vary across species, from mechanical pressure in arthropods to the fertilizing sperm in nematodes, echinoderms and vertebrates (reviewed in Horner and Wolfner, 2008a). Despite differences in trigger, a rise of intracellular calcium levels preceding downstream events is found in most species studied to date (reviewed in Swann and Lai, 2016). In Drosophila egg activation, the intracellular calcium rise is triggered by mechanical cues, which can be pressure exerted by the oviduct or from oocyte swelling in vivo (Heifetz et al., 2001) or in vitro due to osmotic pressure from a hypotonic buffer (Horner and Wolfner, 2008b; Page and Orr-Weaver, 1997). This calcium rise takes the form of a transient calcium wave starting from the pole(s) and traversing the oocyte (Kaneuchi et al., 2015; York-Andersen et al., 2015). This calcium wave is initiated by influx of environmental calcium through Trpm channels in response to the mechanical trigger (Hu and Wolfner, 2019). Further propagation of the calcium wave requires release of internal calcium from stores through the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) calcium channel (Kaneuchi et al., 2015). It remains unclear how the initiation of calcium waves triggers the activation of IP3R during this process.
Phospholipase Cs (PLCs) are membrane-associated enzymes that mediate the cleavage of phospholipids, specifically the cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) to produce diacylglycerol (DAG) and IP3. This reaction is involved in multiple signal transduction pathways (reviewed in Kadamur and Ross, 2013). In mammalian egg activation, a sperm-delivered PLC (PLCζ) is responsible for activating IP3R to start the initial calcium rise (Saunders et al., 2002).
Because the propagation of calcium waves in Drosophila egg activation also requires IP3R, we hypothesized that this is also mediated by PLC and the IP3 pathway. The Drosophila genome encodes three PLCs: No receptor potential A (norpA), Small wing (sl) and Phospholipase C at 21C (plc21C). According to the RNAseq database, all three are expressed in Drosophila ovaries (Leader et al., 2017). It is possible that one or more of the three PLCs is involved in transducing the initial calcium influx signal to the IP3 pathway to allow the calcium wave to propagate. Some PLCs can directly bind to Ca2+ and get activated in response to calcium signals (reviewed in Katan, 1998). All three Drosophila PLCs contain EF hand domains, which can potentially bind Ca2+ and directly transduce the ionic signal to downstream pathways (Lewit-Bentley and Réty, 2000).
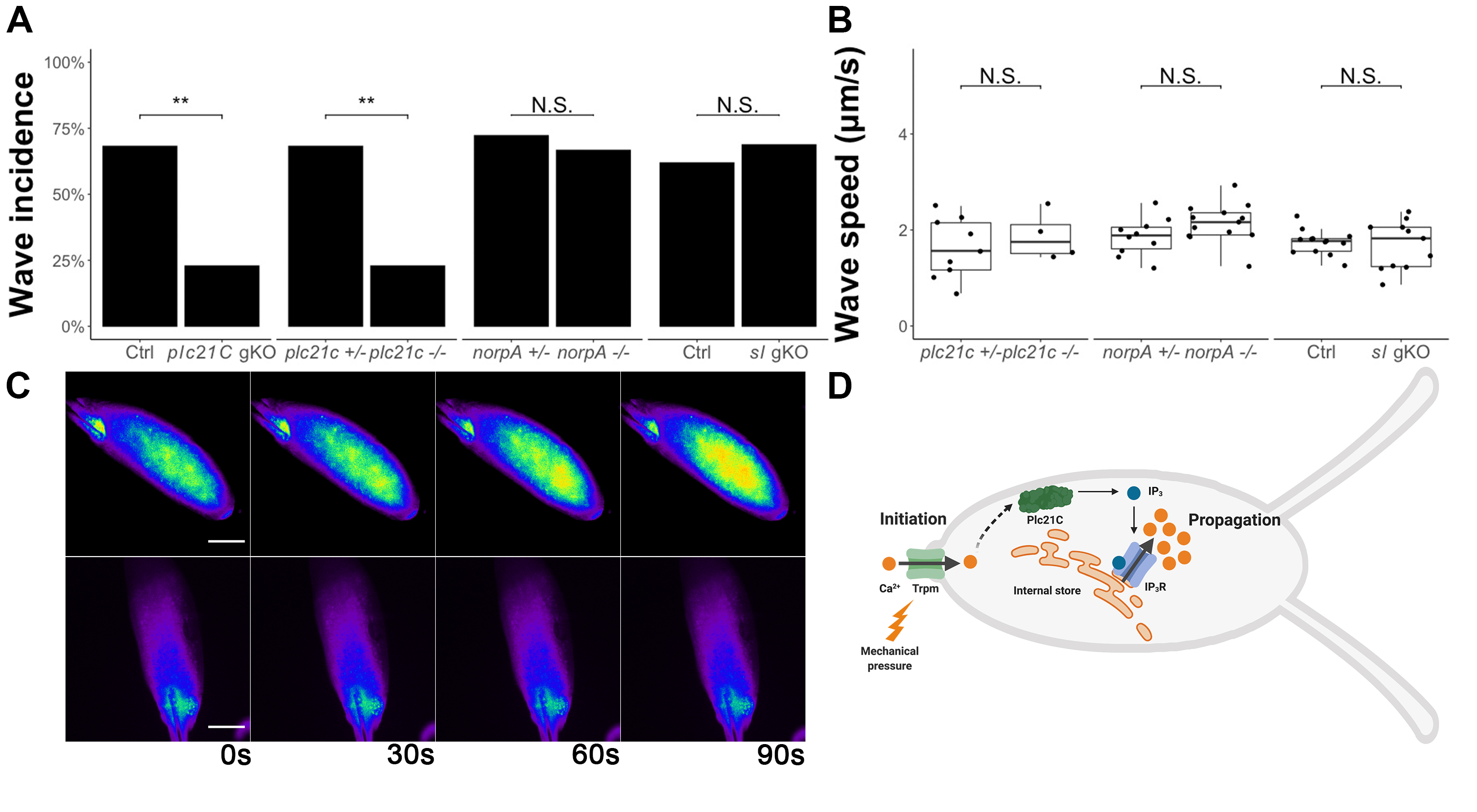
To determine the role of phospholipase C (PLC) in calcium wave propagation during Drosophila egg activation, we screened each of the PLCs. We started by examining the role of Plc21C. We examined the calcium wave phenotype in oocytes from germline-specific CRISPR/Cas9 plc21C knockout females. These females were offspring from nos-Cas9; matα-GAL4-VP16; UAS-GCaMP3 crossed to gRNA-plc21C (see Methods). We observed a significant decrease in calcium wave incidence in oocytes from plc21C germline knockout females compared to controls (Fig.1A). To confirm these results, we isolated a null allele of plc21C (plc21C1) from the offspring of the germline-knockout females (see Methods). This mutation is homozygous viable. We crossed it into the nos-GCaMP6m transgenic background (Hu and Wolfner, 2019) to allow us to visualize calcium dynamics in the germline of plc21C1 females. We examined oocytes dissected from plc21C1 homozygous females during in vitro egg activation and again found a significant decrease in calcium wave incidence compared to heterozygous controls (Fig.1A and C). In the few homozygous plc21C1 oocytes that did show calcium waves, we did not observe a significant difference in calcium wave propagation speed compared to controls (Fig.1B). Taken together, our data show that calcium waves during Drosophila egg activation require Plc21C.
Since there were still calcium waves in a minority of plc21C1 mutant oocytes, we suspected that Plc21C might function redundantly with other molecule(s). We thus examined the role of the two other PLCs encoded by the Drosophila genome, NorpA and Sl. norpA has an available, viable, null allele norpA36 (Riesgo-Escovar et al., 1995). We crossed it into it the nos-GCaMP3-attP2 transgenic background (Kaneuchi et al., 2015) to visualize calcium dynamics in the germline. We isolated mature oocytes from norpA36; nos-GCaMP3-attP2 females and imaged them during in vitro egg activation. We observed that calcium wave incidence and propagation speed did not differ between the oocytes of homozygous norpA36 mutants and heterozygous controls (Fig.1A-B). Next, we examined calcium waves in sl germline knockout oocytes during in vitro activation. Mutant oocytes did not differ from control oocytes in calcium wave incidence or propagation speed (Fig.1A-B). We also isolated a null allele of sl (sl12) from the offspring of sl germline-knockout females (see Methods) and attempted to visualize calcium waves in oocytes from sl12 females. However, sl12 appeared to have combinatorial lethality with the nos-GCaMP6m transgene, as we were unable to isolate homozygous sl12; nos-GCaMP6m flies. The fluorescence signal strength of heterozygous nos-GCaMP6m was too low for us to visualize calcium waves. Although oocytes from germline specific sl knockout females displayed normal calcium wave incidence and propagation speed, it is possible that this knockout did not efficiently cause biallelic null mutations in most oocytes to reveal the function of Sl. Thus, we were unable to determine a requirement of sl for calcium wave propagation.
The presence of calcium waves in a minority of plc21C null oocytes suggests that Plc21C functions redundantly with other PLCs such as Sl or with other calcium signal relaying mechanisms to facilitate calcium wave propagation. These redundant mechanisms require further investigation. It also remains to be elucidated how Plc21C is activated by the initial calcium influx, whether through direct binding of Ca2+ to Plc21C or through other signal relaying molecules. Finally, we note that lack of Plc21C activity did not lead to the presence of initiated but only partially-propagated calcium waves, as was seen for IP3R knockdowns (Kaneuchi et al., 2015). The complete absence of calcium waves seen in most plc21C null oocytes suggests that Plc21C activity is needed at the earliest stages of (or to initiate) wave propagation in response to the Trpm-mediated calcium influx.
In wing imaginal discs, Plc21C is required for the intercellular calcium waves that regulate wing development via the IP3 pathway (Brodskiy et al., 2019). Thus, Plc21C and the IP3 pathway mediate both intracellular calcium waves during egg activation and intercellular calcium waves during tissue development.
This study identified the connection between calcium wave initiation and propagation during Drosophila egg activation. Based on this and our previous studies (Hu and Wolfner, 2019; Kaneuchi et al., 2015), we propose the following model for the mechanism of the calcium wave during Drosophila egg activation: mechanical pressure activates Trpm channels located on the plasma membrane of mature oocytes, allowing influx of external calcium. These Ca2+ ions then directly or indirectly activate Plc21C (and possibly other signal-relaying molecules), which catalyzes the reaction producing IP3. IP3 then binds to and activates its receptor to release calcium from internal stores, facilitating propagation of the calcium wave (Fig.1D). Our demonstration of the use of PLC to relay egg activation triggering signals to intracellular calcium rises reveals an important conservation in egg activation mechanisms between Drosophila and mammals.
Methods
Request a detailed protocolFly strains and maintenance
All Drosophila strains and crosses were maintained or performed on standard yeast-glucose-agar media at 25C° on a 12/12 light/dark cycle. The nos-Cas9; matα-GAL4-VP16; UAS-GCaMP3 transgenic line was made by crossing yw, nos-Cas9 into a previously described matα-GAL4-VP16; UAS-GCaMP3 transgenic line (Kaneuchi et al., 2015). The nos-GCamP3 and nos-GCaMP6m transgenic lines were as previously described (Hu and Wolfner, 2019; Kaneuchi et al., 2015). norpA36 (9048) and yw, nos-Cas9 (54591) fly lines were obtained from the Bloomington Drosophila Stock Center.
DNA constructs and transgenic flies
Calcium waves were visualized by expressing GCaMP calcium sensors in the female germline using matα4-GAL4-VP16; UASp-GCaMP3 or nos-GCaMP6m as previously described (Hu and Wolfner, 2019; Kaneuchi et al., 2015). To generate CRISPR/Cas9 knockouts of plc21C and sl, we followed the previously described germline specific CRISPR/Cas9 genome editing protocol (Hu and Wolfner, 2019; Poe et al., 2018). The offspring of the germline knockout females were isolated and sequenced to establish stable lines of plc21C and sl null mutants. The following gRNA target sequences were used:
gRNA-plc21C: CTACATCTCCACCGCCAGCG; CTTCTGGAACGGACGCACCG
gRNA-sl: ACCATTGGTATGCTGGAGCG; CTCCAGTGAATCCTCCTGCG
These gRNA expression constructs were injected by Rainbow Transgenic Flies, Inc. into yw, nos-phiC31; PBac{attP-9A} embryos. Flies carrying correct insertions were isolated to establish gRNA expression transgenic lines. To generate whole fly knockout strains for plc21C and sl, we crossed germline knockout females to males carrying balancer chromosomes. The F1 progeny were single-pair mated with balancer flies. Once the crosses began producing offspring the parent containing a putative PLC mutation was individually genotyped with PCR. Primers flanking the gRNA targeting sites were used in PCR to detect deletions. Primer sequences are as follows: plc21C-F: TCGGATACCAACCAGGACTATG, plc21C-R: TATCTCGGGCACGAACGTATAG; sl-F: CGGATGAGAACTGGATTCGATAG, sl-R: GTGCAGTATGACAAAGCACTTG. The F2 progeny of crosses from the confirmed-mutant F1s were brother-sister mated to establish stable mutant lines. plc21C1 carries a ~19kb deletion from exon 1 to exon 8, covering the majority the gene. sl12 carries a 44 bp deletion in exon 1 that leads to a frameshift and premature stop codon.
In vitro egg activation assay and imaging
Oocytes were dissected from the indicated female flies fattened on yeast and were induced to activate in vitro following methods as previously described (Hu and Wolfner, 2019; Kaneuchi et al., 2015). Before imaging, oocytes were placed in a drop of Isolation Buffer (IB) (Page and Orr-Weaver, 1997) in a glass-bottomed Petri dish. IB was then replaced by modified Robb’s buffer (RB) (Hu and Wolfner, 2019) to induce egg activation at the start of imaging. Time-lapse images were taken at every 1s for 20 min after the addition of RB, using Zeiss Elyra Super Resolution Microscope with a 10X lens and Zen software. The detection wavelength was set to 493-556 nm, for the GCaMP signal.
Statistics
Pearson’s χ2 test was used to compare the incidence of calcium waves. Student’s t test was used to compare the propagation speeds of the calcium waves.
Acknowledgments
We thank the Society for Developmental Biology’s “Choose Development!” Fellows’ program and Cornell Molecular Biology and Genetics department’s National Science Foundation-funded Research Experience for Undergraduates program for support of ANVA.
References
Funding
NIH grant R21-HD088744 (to MFW). NSF Grant 1428922 funding (for the shared Zeiss Elyra Super Resolution Microscope). For ANVA: partial support from Cornell’s Department of Molecular Biology and Genetics and its NSF-funded REU program (funded on NSF REU DBI-1659534), and partial support from the Society of Developmental Biology’s Choose Development! Program, which is funded by NSF (IOS-1239422; Broadening participation of underrepresented groups in Developmental Biology and REU DBI-1156528).
Reviewed By
Anonymous and Steven MarygoldHistory
Received: February 17, 2020Accepted: March 27, 2020
Published: April 1, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Hu, Q; Vélez-Avilés, AN; Wolfner, MF (2020). Drosophila Plc21C is involved in calcium wave propagation during egg activation. microPublication Biology. 10.17912/micropub.biology.000235. Corrigendum in: microPublication Biology. 10.17912/micropub.biology.000240.Download: RIS BibTeX