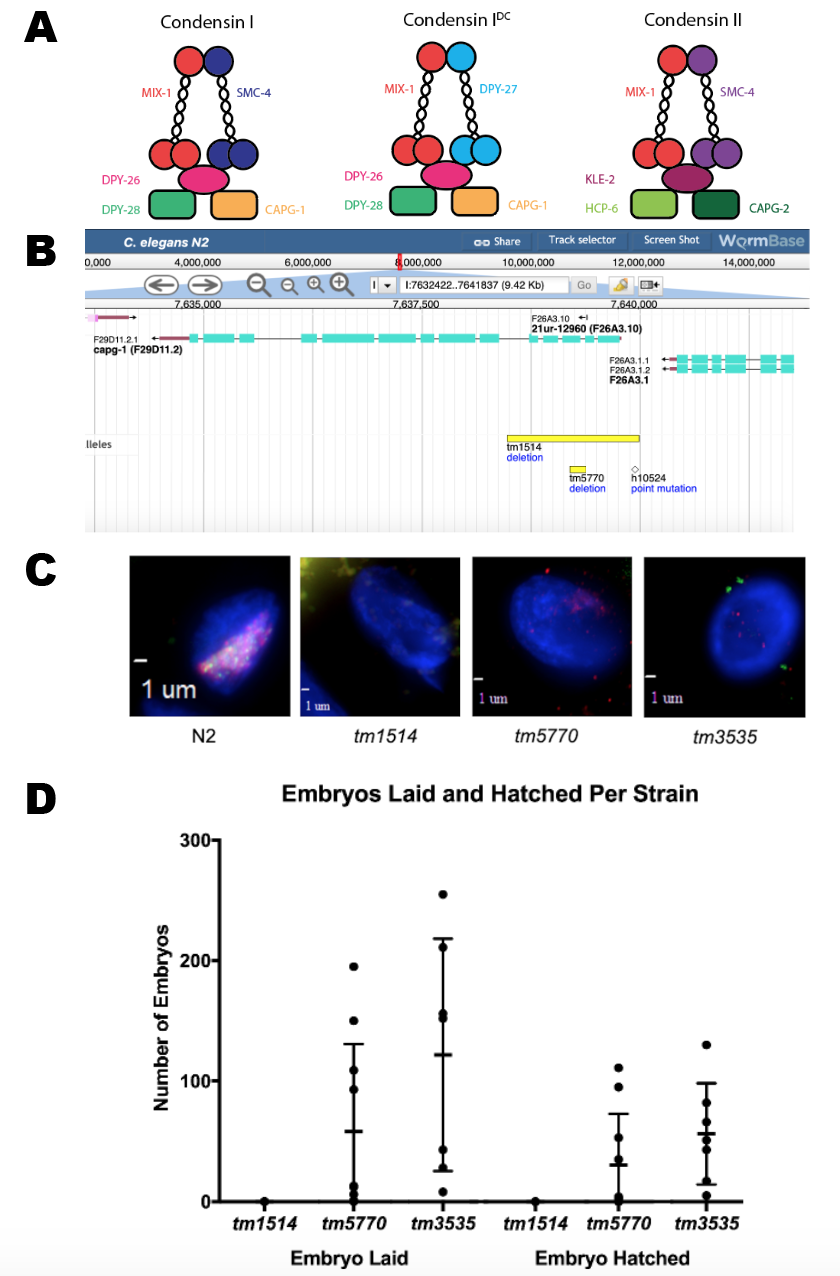
Description
Dosage compensation is the mechanism by which organisms correct the sex chromosome imbalance between sexes (e.g. females having two X chromosomes compared to one X in males). In C. elegans, dosage compensation is achieved by the downregulation by half of both X chromosomes in hermaphrodites (Albritton & Ercan, 2018). This downregulation is accomplished by the Dosage Compensation Complex (DCC), which is comprised of a condensin IDC subcomplex interacting with other accessory proteins. Condensin IDC has a similar structure to canonical condensins (condensin I and condensin II), which function to compact chromosomes during mitosis and meiosis (Csankovszki et al., 2009). The DPY-27 subunit is unique to condensin IDC, MIX-1 is present in all three condensins, while the proteins CAPG-1, DPY-26, and DPY-28 are found both in condensin I and condensin IDC (Figure 1A).
DCC mutants show maternal effect lethality, since all subunits of condensin IDC and several of the accessory proteins are maternally contributed to oocytes (Plenefisch et al., 1989; Csankovszki et al., 2009). Homozygous DCC mutants derived from heterozygous mothers survive to adulthood due to the maternally provided RNA and/or protein. These mutants are referred to as maternal positive, zygotic negative (M+Z-). M+Z- hermaphrodites are unable to produce a functional gene product; therefore, their progeny have no maternal or zygotic contribution of these proteins (M-Z-). As a consequence, very few M-Z- hermaphrodites survive past the L1 stage; however, males do not require the DCC to survive. It is possible, then, to recover M-Z- male progeny from self-fertilizing hermaphrodites in these conditions.
A previous study (Csankovszki et al. 2009) showed that M+Z- capg-1 null mutants (tm1514) are sterile and have severe developmental defects. This phenotype is different from and more severe than what was previously seen for genes encoding other condensin IDC members (Plenefisch et al., 1989). It raised the possibility that the sterility and more severe developmental phenotypes of capg-1(tm1514) is the result of another role of CAPG-1 outside of condensin I and IDC function. We acquired another capg-1 allele (tm5770) from the Japanese National Bioresource Project. This allele deletes a smaller portion of the coding sequence than capg-1(tm1514) but is also predicted to be null due to the resulting frameshift mutation. Also of interest, the capg-1(tm5770) allele removes only the terminal nucleotide from a short piRNA gene deleted entirely in capg-1(tm1514) (Figure 1B). If the absence of the CAPG-1 protein function was responsible for the sterility phenotype observed in capg-1(tm1514), the M+Z- hermaphrodites from the capg-1(tm5770) strain should also be sterile.
We first confirmed via fluorescence microscopy that the DCC is not recruited to the X chromosome in the capg-1(tm1514) adult hermaphrodites, consistent with previously published results (Csankovszki et al., 2009). For additional control, we used a mutation in another condensin IDC member, dpy-28(tm3535), which has similar defects (Hernandez et al., 2018). Fluorescent antibodies specific to CAPG-1 and another condensin IDC subunit, DPY-27, were used to visualize localization of the DCC to the X chromosome compared to wild type (N2) (Figure 1C). N2 hermaphrodites have overlapping signals of CAPG-1 and DPY-27 on both X chromosomes. The X localization of these two condensin IDC proteins is also absent in capg-1(tm5770). This indicates that the capg-1(tm5770) mutation also disrupts DCC localization to the X to a similar degree as capg-1(tm1514) or dpy-28(tm3535).
The capg-1(tm5770) M+Z- hermaphrodites, unlike the capg-1(tm1514) mutants, were observed laying embryos, some of which hatched then arrested in L1, showing more phenotypic similarity to the dpy-28(tm3535) mutants than the capg-1(tm1514) mutants. To quantify this observation, we conducted embryo and lethality counts in capg-1(tm1514), capg-1(tm5770), and dpy-28(tm3535) mutants to assess both the number of embryos laid and the number of embryos hatched (Figure 1D). Our results show that while capg-1(tm1514) M+Z- mutants did not lay any eggs, the capg-1(tm5770) M+Z- and the dpy-28(tm3535) M+Z- mutants produced significant numbers of embryos. Many of the embryos laid by the capg-1(tm5770) and dpy-28(tm3535) mutants hatched then arrested in the L1 stage. Interestingly, there is a high amount of variability in numbers of embryos laid between individual worms in the dpy-28(tm3535) and capg-1(tm5770) strains. The dpy-28(tm3535) and capg-1(tm5770) mutants produced a small percentage of M-Z- progeny that survived until adulthood. Phenotypically, these were either males or very Dpy hermaphrodites that had severe developmental defects and were sterile. The capg-1(tm5770) mutants (n=10) laid an average of 58 embryos per worm, ranging between 0 and 195. Of the 580 total embryos laid, 297 hatched, of which 291 arrested in the L1 stage, with 1 male and 5 hermaphrodites surviving to adulthood. The dpy-28(tm3535) mutants (n=7) laid an average of 122 embryos per worm, ranging between 8 and 255. Of the 853 total embryos laid, 354 hatched, of which 340 arrested in the L1 stage, with 9 males and 5 hermaphrodites surviving to adulthood. The appearance of males in the M-Z- progeny is consistent with a weak Him phenotype reported previously for condensin I mutants (Plenefisch et al., 1989; Hernandez et al., 2018). Overall, these results indicate that the capg-1(tm5770) mutation results in phenotypes resembling the phenotypes caused by dpy-28(tm3535) and other mutations in condensin IDC subunits (Plenefisch et. al, 1989). These condensin IDC mutant phenotypes are different from the phenotypes resulting from the capg-1(tm1514) mutation.
These data suggest that the more severe phenotype in the capg-1(tm1514) mutants is not due to disruption of CAPG-1 function. There are several potential alternative explanations. The phenotype may be due to the deletion of the piRNA gene near the 5’ end of the capg-1 gene (Figure 1B). It is also possible that the severe phenotype observed in capg-1(tm1514) is due to a disruption of the trans-splice site between genes. The capg-1 gene is last in its operon, and the capg-1(tm1514) deletion includes a trans-spliced acceptor site (Worm Base). This would result in defective trans-splicing between capg-1 and the upstream gene, F26A3.1. Our data does suggest, however, that the severity of the capg-1(tm1514) phenotype is not due to an alternative role of CAPG-1 outside of condensin I and condensin IDC function.
Methods
Request a detailed protocolStrains: All C. elegans strains were maintained using standard methods and fed E. coli (OP50) on NG agar plates and maintained at 20oC. The strains used included N2 Bristol strain (wild-type) as a negative control, EKM4 capg-1(tm1514) I/hT2 [qIs48] (I;III), EKM86 capg-1(tm5770) I/hT2[qIs48] (I;III), and EKM40 dpy-28(tm3535) III/hT2[qIs48] (I;III). M+Z- hermaphrodites were identified by selecting GFP-negative progeny of GFP-positive hermaphrodites.
Immunofluorescence Imaging: Young adult worms were dissected with needles in 10μL of 1X sperm salts (50mM Pipes pH7, 25 mM KCl, 1 mM MgSO4, 45 mM NaCl, with 1 mM levamisole as a sedative), fixed in 2% paraformaldehyde in 1X sperm salts for five minutes in a humid chamber moistened with PBST (PBS with .1% Triton X-100), and frozen on dry ice with a coverslip for at least 15 minutes. After freezing, the coverslip was carefully separated with a razor blade and the slides were washed three times for 10 minutes each in PBST. This was followed by overnight incubation in a humid chamber with 40μL of a solution of primary antibodies rabbit anti-DPY-27 and rat anti-CAPG-1 (Csankovszki et al., 2009) diluted 1:250 in PBST. One primary antibody targeted DPY-27, which is part of Condensin IDC in the Dosage Compensation Complex, and was raised in rabbit (Csankovszki et al., 2009). The other primary antibody targeted CAPG-1, which is part of both Condensin I and Condensin IDC, and was raised in rat. Incubation with primary antibody was overnight in a humid chamber at room temperature. The next day, slides were washed three times for 10 minutes each in PBST, incubated for 1 hour at 37oC with 30μL of a solution of secondary antibody (Jackson Immunochemicals Cy3 conjugated anti-rabbit for DPY-27 and FITC conjugated anti-rat for CAPG-1 at 1:100), and washed again three times for 10 minutes each in PBST with the final wash containing 1uL of DAPI (1mg/mL). Slides were then mounted with Vectashield (Vector Laboratories).
Acknowledgments
We would like to thank the Japanese Bioresource Project for providing the capg-1(tm1514), capg-1(tm5770), and dpy-28(tm3535) strains. We would also like to thank the members of the Csankovszki Lab for their helpful discussions.
References
Funding
This work was supported by NSF grant MCB 1923206.
Reviewed By
AnonymousHistory
Received: April 1, 2020Revision received: May 5, 2020
Accepted: May 5, 2020
Published: May 10, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
VanDiepenbos, S; Csankovszki, G (2020). Difference in phenotypic severity of presumed null alleles of capg-1. microPublication Biology. 10.17912/micropub.biology.000245.Download: RIS BibTeX