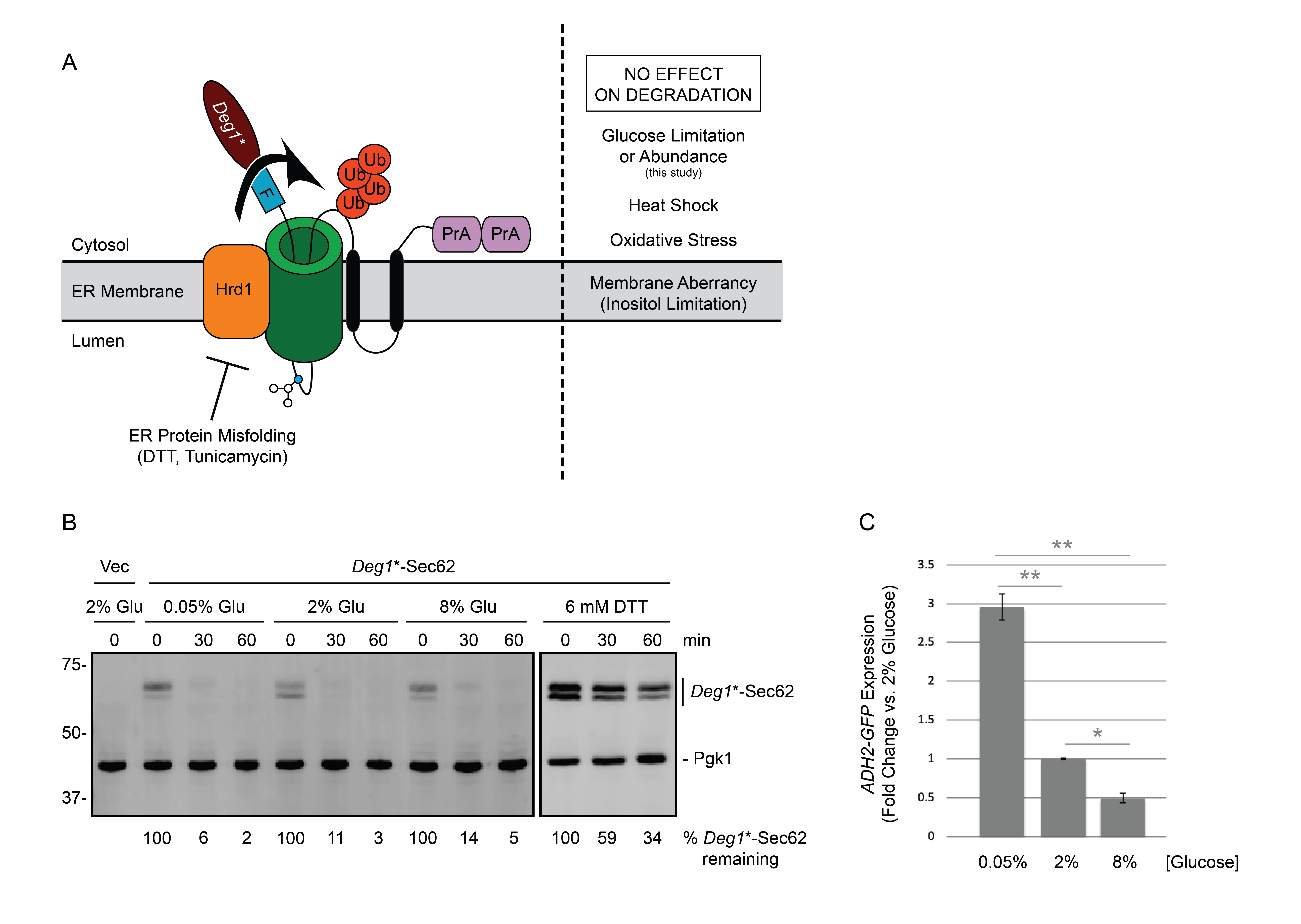
Description
Approximately one third of eukaryotic proteins enter the endoplasmic reticulum (ER) en route to their subcellular or extracellular destinations (Chen et al. 2005; Choi et al. 2010). Many of these proteins use the Sec61p translocon complex to cross the ER membrane (Aviram and Schuldiner 2017). Proteins that persistently engage the translocon prevent other proteins from reaching the ER (Izawa et al. 2012; Ast et al. 2016). Thus, cells have evolved multiple quality control mechanisms to degrade proteins that aberrantly occupy this channel (Rubenstein et al. 2012; Crowder et al. 2015; Ast et al. 2016). In ER-associated degradation of translocon-associated proteins (ERAD-T), such polypeptides are targeted for destruction by homologs of the ER-resident RING (really interesting new gene) domain ubiquitin ligase Hrd1p. Deg1*-Sec62 is an engineered model translocon-associated substrate for Hrd1p in yeast (Figure 1A). Analogously, in mammalian cells, the Hrd1p homolog gp78 promotes turnover of the low-density lipoprotein (LDL) component apolipoprotein B, which stalls in the translocon if it is unable to associate with LDL lipid molecules (Fisher et al. 2011).
We recently discovered that degradation of Deg1*-Sec62 is impaired by ER stress (the accumulation of misfolded or unfolded proteins in the ER). Deg1*-Sec62 is strongly stabilized by treatment with dithiothreitol (DTT; which reduces disulfide bonds) or tunicamycin (which prevents N-linked glycosylation). By contrast, however, Deg1*-Sec62 degradation is unaffected by perturbation of ER membrane lipid composition (i.e. inositol limitation) or treatments expected to broadly perturb proteostasis (elevated temperature or oxidative stress) (Buchanan et al. 2019).
The AMP-activated protein kinase Snf1p is stimulated during ER stress (Mizuno et al. 2015). Further, loss of the Snf1p inhibitor Reg1p renders cells hypersensitive to ER stress (Ferrer-Dalmau et al. 2015). Snf1p is also regulated by nutrient abundance; it is activated by phosphorylation when glucose is limiting and inactivated by dephosphorylation when glucose is abundant (Rubenstein et al. 2008). Given ERAD-T sensitivity to ER stress and crosstalk between ER stress and nutrient stress signaling, we sought to determine if turnover of the ERAD-T substrate Deg1*-Sec62 is regulated by changes in glucose abundance.
We performed cycloheximide chase experiments to compare Deg1*-Sec62 degradation kinetics in low (0.05%), standard (2%), or high (8%) glucose concentrations (Figure 1B). Deg1*-Sec62 was rapidly degraded in all three conditions. By contrast, DTT strongly stabilized and impaired post-translational modification of Deg1*-Sec62, as previously reported (Buchanan et al. 2019). ADH2 expression is repressed by glucose (Dombek et al. 1993). To confirm differences in glucose abundance, ADH2-GFP expression was compared using flow cytometry of a parallel culture (Figure 1C). Our results indicate that changes in glucose abundance (in the range of 0.05% to 8%) do not substantially alter the rate of degradation of Deg1*-Sec62, a model translocon-associated substrate of Hrd1p.
Taken with our recently published work (Buchanan et al. 2019), our results indicate that ERAD-T is inhibited by stress caused by ER protein misfolding but not membrane stress, oxidative stress, heat shock, or glucose limitation or abundance. It remains possible that altered glucose levels exert an effect on ERAD-T in the context of ER stress or mutations in genes mediating crosstalk between ER stress and nutrient signaling. Future experiments may be performed to test these hypotheses. During ER stress, protein translocation into the ER is slowed (Kang et al. 2006). We speculate that inhibited degradation of proteins that persistently engage the translocon contributes to reduced overall rates of translocation, preventing an already stressed ER from becoming overwhelmed.
Methods
Request a detailed protocolYeast and Plasmid Methods
Yeast were cultured at 30°C in synthetic-defined growth media (Guthrie and Fink 2004). An empty vector (pVJ27/pRS316; URA3/CEN (Sikorski and Hieter 1989)) and a plasmid encoding Deg1*-Sec62 driven by the MET25 promoter (pVJ317; URA3/CEN (Rubenstein et al. 2012)) were introduced to yeast (VJY476/BY4741 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 (Tong et al. 2001)) via lithium acetate transformation (Guthrie and Fink 2004). Yeast expressing ADH2 with a C-terminal GFP tag (VJY731; MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ADH2-GFP:HIS3MX6) were obtained from the Yeast GFP Clone Collection (Invitrogen (Huh et al. 2003)).
Flow Cytometry
Yeast expressing ADH2-GFP were cultured, in triplicate, to mid-exponential growth at 30°C in media containing 2% glucose, washed five times in media containing 0.05%, 2%, or 8% glucose, and incubated in fresh media containing the same glucose concentrations for two hours, as indicated. Mean GFP fluorescence of 10,000 cells was measured using the MACSquant Analyzer X.
Cycloheximide Chase Analysis, Cell Lysis, and Western Blotting
Cycloheximide chase analysis was performed as described previously (Buchanan et al. 2016). For glucose treatments, yeast cultured to mid-exponential phase growth in media containing 2% glucose were washed five times in media containing 0.05%, 2%, or 8% glucose and incubated in fresh media containing the same glucose concentrations for two hours at 30°C. For cultures treated with dithiothreitol (DTT), DTT was added to mid-exponential phase cultures (6 mM DTT final concentration) for one hour of incubation at 30°C. Glucose and DTT concentrations were maintained throughout the course of the cycloheximide chase. Proteins were extracted and analyzed by western blotting as described previously (Kushnirov 2000; Watts et al. 2015). Deg1*-Sec62 is C-terminally tagged with two copies of the Staphylococcus aureus protein A epitope (Figure 1A). S. aureus Protein A binds to mammalian immunoglobulins (Hjelm et al. 1972); therefore, AlexaFluor-680-conjugated rabbit anti-mouse antibody (Life Technologies, Inc; 1:40,000) was used to directly detect Deg1*-Sec62. Pgk1p was detected with mouse anti-phosphoglycerate kinase 1 (Pgk1; clone 22C5D8; Life Technologies, Inc; 1:20,000) followed by AlexaFluor-680-conjugated rabbit anti-mouse secondary antibody (1:40,000). Membranes were imaged and analyzed using an Odyssey CLx Infrared Imaging System and Image Studio Software (Li-Cor).
Acknowledgments
We thank Martin Schmidt for insightful conversations and ongoing moral support. We thank Kyle Richards for critical reading of this manuscript. We thank Kelsey Woodruff and Seth Horowitz for laboratory assistance during the project.
References
Funding
This work was funded by a Ball State University ASPiRE Graduate Student Research Grant (CLB) and NIH grant R15 GM111713 (EMR).
Reviewed By
AnonymousHistory
Received: April 2, 2020Revision received: May 5, 2020
Accepted: May 8, 2020
Published: May 8, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Broshar, CL; Rubenstein, EM (2020). Glucose concentration does not affect degradation of a protein that aberrantly engages the endoplasmic reticulum translocon. microPublication Biology. 10.17912/micropub.biology.000248.Download: RIS BibTeX