Current address: Lunenfeld-Tanenbaum Research Institute, Sinai Health System, 600 University Avenue, Toronto, ON M5G 1X5, Canada
Description
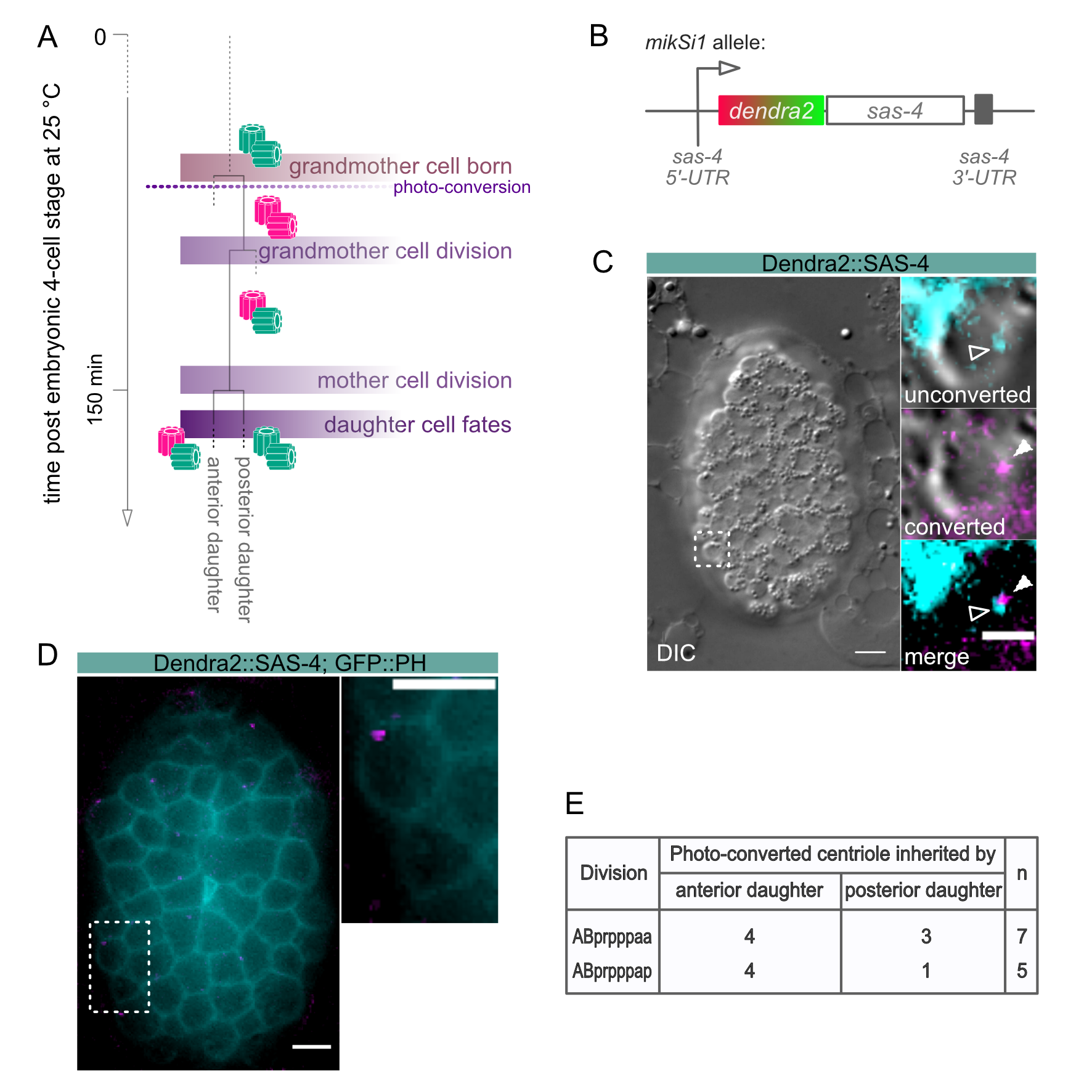
The nematode C. elegans possesses a relatively small subset of centrosome proteins and hence, has emerged as an important model system in elucidating mechanisms of centrosome biogenesis and dynamics. The most basic factors of the centrosome assembly pathway were discovered and characterized in the worm. The centrosome consists of a pair of centrioles, surrounded by the pericentriolar material, and its duplication is strictly coupled to the cell cycle. In worms, the centriole duplication pathway comprises the protein SPD-2Cep192, which recruits the kinase ZYG-1PLK4 to centrioles to initiate centriole assembly (O’Connell et al., 2001; Kemp et al., 2004; Pelletier et al., 2004). ZYG-1PLK4 in turn, recruits SAS-6hsSAS6, which in complex with SAS-5STIL triggers the formation of the central tube (Dammermann et al., 2004; Delattre et al., 2004; Leidel et al., 2005; Kitagawa et al., 2009; Qiao et al. 2012; Hilbert et al., 2013; Lettman et al., 2013; Rogala et al., 2015). Subsequently, the coiled-coil protein SAS-4CPAP is stably incorporated into the centriole wall and assembles singlet microtubules around the forming centriole (Kirkham et al., 2003; Leidel and Gönczy, 2003, Balestra et al., 2015). SAS-7Cep295 is required for paddlewheel structure formation and recruits SPD-2Cep192 for a new round of centriole formation in the following cell cycle (Chang et al., 2016; Saurya et al., 2016; Sugioka et al., 2017). Due to the nature of the duplication of centrioles, there is always one older and one younger centriole present in a centriolar pair. Hence, in the subsequent cell division, daughter cells will inherit centrosomes carrying mother centrioles of different ages. Studies in Drosophila melanogaster and mammals have shown that mother centrosome and daughter centrosome can be segregated in a non-random manner during stem cell divisions and that this segregation pattern correlates with the fates of the daughter cells (Yamashita et al., 2007; Wang et al., 2009; Conduit et al., 2010; Januschke et al., 2011). Due to the invariant cell lineage of C. elegans and the possibility to follow individual cell fates, tracking centriole inheritance in regard to their age in worms could provide valuable information about the impact of centriole age on differentiation (Sulston and Schierenberg, 1983). However, factors that localize to one of the two centrioles in an age-dependent manner have not been identified in C. elegans to date, making it impossible to distinguish older from younger centrosomes in worms. To overcome the limitation of following age-related centrosome inheritance in C. elegans, we generated a strain in which centrioles are labeled with a photo-switchable marker. Once centrioles are photo-converted, they can be tracked over several cell cycles, and centrosome age can be distinguished after the second round of duplication (Figure 1A). The photo-switchable fluorescent protein Dendra originates from the octocoral Dendronephthya sp. The protein can be irreversibly converted from a green-to-red fluorescence state by exposure to visible blue or ultraviolet light. In this study, we made use of the bright and fast-maturing Dendra2 version of the protein, which was fused to the centriolar protein SAS-4CPAP and expressed under endogenous regulatory sequences (Figure 1 B, Gurskaya et al., 2006, Ihara et al., 2011). SAS-4CPAP is stably incorporated into centrioles and shows no cytoplasmic exchange once centrioles are formed (Dammermann et al., 2004, Balestra et al., 2015). We did not notice any significant difference in embryonic lethality of the strain at 25°C. On average we found 1.4% embryonic lethality for sas-4p::dendra2::sas-4 (n=1322 embryos); in comparison to 1.1% for a wild-type control strain (n=1692 embryos). In this study, we show that the older and younger centrosome can be successfully distinguished within cells of different lineages in C. elegans (Figure 1 C).
L4 larvae were grown at 25°C overnight to adulthood. The next day, worms were dissected to collect embryos shortly after fertilization in H2O on a coverslip (Carl Roth GmbH; 18 x 18 mm, #1 thickness; Cat. no. 0657.2). Embryos were reversely mounted on a 4 % agarose pad on a microscope slide and sealed with petroleum jelly. The development of embryos starting at 4-cell stage was followed under a 4D-microscope at 25°C. The lineaging of the cells of interest was performed simultaneously while recording. Images were taken using a Zeiss Axio Imager.M2 equipped with epifluorescence and the Time to Live software from Caenotec. Differential interference contrast (DIC) micrograph Z-stacks were taken every 35 sec at 25°C. Fluorescent scans were taken as required. The Simi BioCell software was used for the lineage analysis (Simi Reality Motion Systems GmbH; http://www.simi.com) as previously described (Schnabel et al., 1997). Embryos were allowed to develop until shortly after the onset of the ABprppp cell division. Subsequently, embryos were exposed to UV light on a Zeiss Axioscop 2 microscope for photo-conversion of the Dendra2 fluorophore (conversion time: 15-17 sec; whole embryos were exposed to UV light, and all centrioles present at this stage were photo-converted). All centrioles formed prior to photo-conversion display red fluorescence (magenta) after the exposure to UV light, whereas centrioles formed after conversion display green fluorescence (cyan; Figure 1 A). Lineaging of the embryos was continued on the 4D microscope after photo-conversion.
As proof of principle, we analyzed the age-dependent inheritance pattern of centrioles in a strain expressing Dendra2::SAS-4, as well as GFP::PH, to reliably mark the outlines of individual cells. The cell lineage chosen was still easy to track but close to the final differentiation state. Centrioles were photo-converted in the grandmother cell ABprpppa (Figure 1 A). The divisions of the daughter cells ABprpppaa and ABprpppap were followed to determine the segregation of the centrioles (Figure 1 D and E). Fluorescence images were taken after the divisions of the sister cells. The ABprpppaa daughter cell divides into equally sized granddaughter cells. Here, in 57 % of the divisions, the older converted centriole is inherited by the anterior cell, and in 43 % of the divisions by the posterior cell (n=7, Figure 1 E). The granddaughter cells deriving from the ABprpppap cell division are unequal in size, with the posterior granddaughter being smaller and fated to die. In 80 % of the divisions, the older converted centriole segregates into the anterior cell. In the remaining 20 % of the cases, the posterior cell inherits the converted centriole (n=5, Figure 1 E). Thus, despite what fate the granddaughter cells adapt, centrioles are segregated randomly in both lineages. Taken together, our approach allows the successful tracking of centrosome inheritance in the invariant divisions of the C. elegans lineage.
Methods
Request a detailed protocolWe applied standard methods for DNA amplification, analysis, and manipulation. For PCR amplification, the Phusion® High-Fidelity DNA Polymerase (New England Biolabs) was used according to the manufacturer’s protocol. The sas-4 sequence and regulatory regions were amplified from C. elegans genomic DNA and pAD154. The dendra2 nucleotide sequence was introduced upstream of the sas-4 coding sequence in the MosSCI vector pCFJ350 to generate the TMD29 [sas-4p::dendra2::sas-4::sas-4] plasmid (Figure 1 B). Cloning was performed via the sequence and ligation independent cloning (SLIC) method (Jeong et al., 2012) using the T4 DNA polymerase (New England Biolabs) and NEBuffer 2.1 (New England Biolabs). Sanger sequencing was applied to analyze DNA sequences. The TMD29 plasmid was integrated into the second chromosome of the C. elegans genome by the universal MosSCI single-copy integration method (Frøkjær-Jensen et al., 2014, integration strain: EG6699 [ttTi5605; unc-119(ed3)]) to generate the mikSi1 allele. Germline microinjection was performed as described by Mello et al.. (1991) (Mello and Kramer, 1991). Image analyses were performed with Fiji/ImageJ 2.0.0 (Schindelin et al., 2012, https://fiji.sc/). To test for embryonic lethality, singled L4 worms were allowed to lay eggs at 25°C overnight. Total embryos laid and hatched worms were scored in three independent experiments. The N2 Bristol strain was used as control.
Reagents
Nematode strains:
N2 C. elegans wild isolate
TMD42 mikSi1[sas-4p::dendra2::sas-4]II; unc-119(ed3)III
TMD57 mikSi1[sas-4p::dendra2::sas-4]II; bcIs57[pie-1p::gfp::plcδph]
Nematode strains were maintained at 15 °C under standard conditions (Brenner, 1974).
TMD57 will be made available on CGC.
Acknowledgments
The authors wish to thank N. Memar for help with 4D-lineaging; M. Schwarz and N. Lebedeva for excellent technical support. We thank D. Sherwood and A. Dammermann for providing the Dendra2 and SAS-4 sequences. We thank C. Nöcker for performing the embryonic lethality test. Some strains used in this study were provided by the Caenorhabditis Genetic Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by DFG MI 1867/1-1 to TMD.
References
Funding
DFG MI 1867/1-1
Reviewed By
Jyoti Iyer and AnonymousHistory
Received: March 12, 2020Revision received: April 18, 2020
Accepted: May 17, 2020
Published: May 28, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Erpf, AC; Mikeladze-Dvali, T (2020). Tracking of centriole inheritance in C. elegans. microPublication Biology. 10.17912/micropub.biology.000256.Download: RIS BibTeX