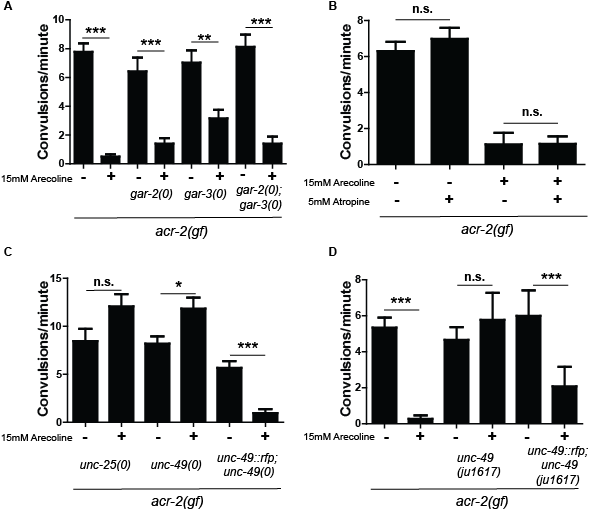Description
acr-2 encodes a nicotinic receptor subunit. A gain-of-function mutation in acr-2 results in uncoordinated (Unc) movement and spontaneous shrinking or convulsion (Jospin, 2009). These phenotypes are the result of elevated cholinergic excitation as well as decreased GABAergic inhibition in the locomotor circuit. We have tested the effect of the muscarinic agonist arecoline on acr-2(gf) convulsion and found that this drug suppressed convulsion and locomotion phenotypes (McCulloch and Jin 2020). Here, we investigated underlying mechanisms and pathways involved in arecoline-induced suppression of acr-2(gf).
There are two major types of muscarinic receptors. The M1/3/5 types are excitatory and act by elevating intracellular calcium. The M2/4 receptors are inhibitory and act by inhibiting cellular cAMP (Jones et al. 2012). C. elegans has three muscarinic receptors: gar-1, gar-2 and gar-3 (Hwang et al. 1999; Lee et al. 2000). Both gar-2 and gar-3 have been shown to respond to arecoline in vivo. gar-3 is homologous to excitatory M1 muscarinic receptors, and promotes excitatory signaling in pharyngeal and motor circuits (Chanet al. 2013; Hwang et al. 1999; Kozlova et al. 2019; Liu et al. 2007; Steger and Avery 2004). gar-2 is pharmacologically quite different from most muscarinic receptors, however, functionally, it seems to act similarly to inhibitory M2/4 type receptors in inhibiting cholinergic activity in the motor circuit (Dittman and Kaplan 2008; Lee et al. 2000). gar-1, unlike gar-2 and gar-3, is expressed in the PVM neurons and other neurons in the head and has not been shown to be expressed in the motor circuit (Lee et al. 2000). We therefore focused on gar-2 and gar-3. We constructed double and triple mutants of acr-2(gf) with null mutations in gar-2 or gar-3 (or mutations of both, respectively). Without arecoline, these mutants showed convulsion phenotypes indistinguishable from acr-2(gf) alone. Upon treatment with arecoline, we observed suppression of convulsion, similar to acr-2(gf) alone (Figure 1A). These genetic data suggested that these known muscarinic receptors in the motor circuit are not involved in suppression of acr-2(gf) by arecoline.
To further address if suppression of acr-2(gf) by arecoline involves muscarinic signaling, we tested atropine, which is a highly specific muscarinic antagonist and used to delineate muscarinic from non-muscarinic cholinergic effects (Rang, et al. 2012). It is used intravenously to treat patients with dangerously low heart rate, as inhibitory M2 receptors function in the heart to regulate cardiac activity. We treated acr-2(gf) animals with 5mM atropine for 2 hours, then transferred the animals to new plates with 5 mM atropine plus 15 mM arecoline for another 3 hrs and score them for convulsion. Atropine only, arecoline only, and untreated acr-2(gf) mutant animals were also scored as controls. However, atropine treatment did not alter the suppression of acr-2(gf) convulsion by arecoline (Figure 1B). This further supports a non-muscarinic mechanism for arecoline suppression of acr-2(gf).
Finally, reverse and forward genetic screens were employed to identify factors that are required for suppression of acr-2(gf) on arecoline. We tested multiple genes that are required for motor circuit function for their requirement for arecoline suppression. We found that null mutations in genes required for GABA signaling, such as unc-25/GAD and unc-49/GABAR, completely blocked arecoline suppression of acr-2(gf) (Figure 1C) (Bamber et al. 1999; Jin et al. 1999). Moreover, convulsion rates of these double mutants were slightly enhanced on arecoline. Null mutations in GABA genes strongly enhance the Unc phenotype of acr-2(gf) animals to paralysis, and this phenotype was also not suppressed on arecoline in the double mutants. These observations suggested that GABA signaling may be important for suppression of acr-2(gf) by arecoline.
In parallel to the reverse genetic screen, we conducted a forward genetic screen to identify factors involved in arecoline suppression of acr-2(gf) (see Reagents and Methods). We isolated three mutants that showed paralyzed phenotypes resembling GABA mutants, with or without arecoline treatment. Non-complementation analyses confirmed two of these to be unc-49 mutations. Another mutation, ju1617, fully blocked arecoline suppression. However, ju1617; acr-2(gf) double mutants resembled acr-2(gf) in the absence of arecoline, in contrast to the unc-49 null mutations. We isolated ju1617 on its own, and these animals were slow-moving and exhibited loopy movement, somewhat similar to GABA mutants. After whole-genome sequencing analyses, we found a single nucleotide mutation altering a conserved Arg to His within a putative ligand-binding domain of UNC-49B (Bamber et al. 1999). To confirm the identity of ju1617, we constructed a ju1617; acr-2(gf) strain with an unc-49 transgene krSi2[Punc-49::unc-49::rfp] and observed that these animals showed suppression of convulsion after arecoline treatment (Figure 1D). The unc-49(ju1617) mutation may represent a reduction-of-function mutation of unc-49, since it does not shrink in response to touch and fails to enhance acr-2(gf) Unc behaviors.
In summary, our studies from both forward and reverse genetic screens indicate that GABA signaling is required for suppression of acr-2(gf) by arecoline. One explanation is that arecoline is activating GABA signaling via an un-identified pathway to restore excitation and inhibition balance to the motor circuit, in the presence of acr-2(gf). Together, these data suggest that arecoline can act through novel means to inhibit cholinergic activity in specific contexts.
Methods
Request a detailed protocolDrug plates were prepared by supplementing standard NGM plates with drugs essentially as described for other C. elegans pharmacology assays (Mahoney et al. 2006). Drugs were dissolved in NGM at indicated concentrations prior to pouring. NGM-only plates were used as no-drug controls. All plates were seeded with a thin lawn of OP50 bacterial food. Two trials were performed for each experiment, with 10 animals in each trial (muscarinic agonists often drive animals to crawl off the plates, so some trials had <10 animals). Animals were transferred to drug plates and then scored after 3 hours. Convulsions were counted over 90s, and then normalized to 60s as convulsions per minute. For atropine studies, animals were incubated on NGM plates supplemented with 5mM atropine and scored for convulsion, or transferred to plates with 5mM atropine and 15mM arecoline, incubated for an additional 3hrs, and then scored for convulsion.
Genetic screening was performed using MT6241, essentially as described with modifications for drug-based isolation of mutants (Brenner 1974; McCulloch et al. 2017). F2s from mutagenized P0s were washed onto the center of 5mM arecoline plates and incubated for 3 hrs. Animals that had not moved from the center spot and visibly convulsed were picked, and then animals were analyzed in a second round, leaving 14 viable suppressors. Whole-genome sequencing analysis was performed as described (McCulloch et al. 2017).
Reagents
STRAINS
MT6241 acr-2(n2420gf) X
CZ9381 unc-25(n2328) III; acr-2(n2420gf) X
CZ9307 unc-49(e382) III; acr-2(n2420gf) X
CZ9364 gar-2(ok520) III; acr-2(n2420gf) X
CZ24303 gar-3(gk305) V; acr-2(n2420gf) X
CZ25223 gar-2(ok520) III; gar-3(gk305) V; acr-2(n2420gf) X
CZ26714 unc-49(ju1617) III
CZ27187 krSi2[Punc-49::unc-49::rfp] II; unc-49(e382) III; acr-2(n2420gf) X
CZ26795 unc-49(ju1617) III; acr-2(n2420gf) X
CZ27978 krSi2[Punc-49::unc-49::rfp] II; unc-49(ju1617) III; acr-2(n2420gf) X
Drugs used in this study:
Arecoline hydrobromide, Acros Organics Cat#AC250130050
Atropine sulfate, Sigma Cat#A0257-256
Acknowledgments
The authors would like to thank Dr. Salvatore Cherra and members of the Jin and Chisholm Labs for helpful discussions. We thank Jean-Louis Bessereau for krSi2, and the CGC for gar-2 and gar-3 strains. We would also like to thank Bhavika Anandpura for assistance in characterizing the arecoline suppression phenotype and ju1617.
References
Funding
K.A.M. was partly supported by a NIH institutional training grant, T32 AG000216. This work is supported by a NIH grant, R37 NS 035546 to Y. J.
Reviewed By
AnonymousHistory
Received: June 15, 2020Revision received: June 26, 2020
Accepted: June 28, 2020
Published: July 1, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
McCulloch, KA; Jin, Y (2020). Novel actions of arecoline in the C. elegans motor circuit. microPublication Biology. 10.17912/micropub.biology.000275.Download: RIS BibTeX