Description
The ability of developing seedlings to respond and adapt to diverse environmental conditions including light is critical for their emergence and establishment (Benvenuti et al., 2001; Forcella et al., 2000; Salter et al., 2003; Yu and Huang, 2017). Cell expansion within the hypocotyl optimizes light and energy capture by the cotyledons, and enables the transition to autotrophic status (Botterweg-Paredes et al., 2020; Dowson‐Day and Millar, 1999; Oh et al., 2013). Hypocotyl elongation is regulated by multiple factors including temperature, phytohormones, circadian clock and light (Dowson‐Day and Millar, 1999; Ma et al., 2016; Procko et al., 2014; Reed et al., 2018; Yu and Huang, 2017). Endogenous and exogenous sugars are also important regulators of hypocotyl cell expansion (Lilley et al., 2012; Liu et al., 2011; Pfeiffer and Kutschera, 1995; Simon et al., 2018b; Singh et al., 2017; Zhang et al., 2010, 2015). Under constant darkness, the interaction between plant hormones such as brassinosteroid and gibberellin and sugar signalling is proposed to stimulate increase in hypocotyl length (Simon et al., 2018b; Zhang et al., 2010, 2015). Hypocotyl phenotypes are important tools in plant biology as they have been used to screen for mutants with altered responses to light and sugar signalling and some of the identified genes have revolutionized plant research (Nakano, 2019). In multicellular organisms, actomyosin-dependent transport constitutes an essential component of the cellular structure and dynamics (Duan and Tominaga, 2018; Kurth et al., 2017; Peremyslov et al., 2010). The Arabidopsis genome encodes 17 myosins classified into two distantly-related groups comprising 4 class VIII myosins and 13 class XI plant myosins (Haraguchi et al., 2019; Reddy and Day, 2001; Ryan and Nebenführ, 2018). The plant-specific-myosin XI has been implicated in diverse developmental processes (Cai et al., 2014), but the contribution of members of class VIII myosins including myosin1/ATM1 to plant development stills remains elusive.
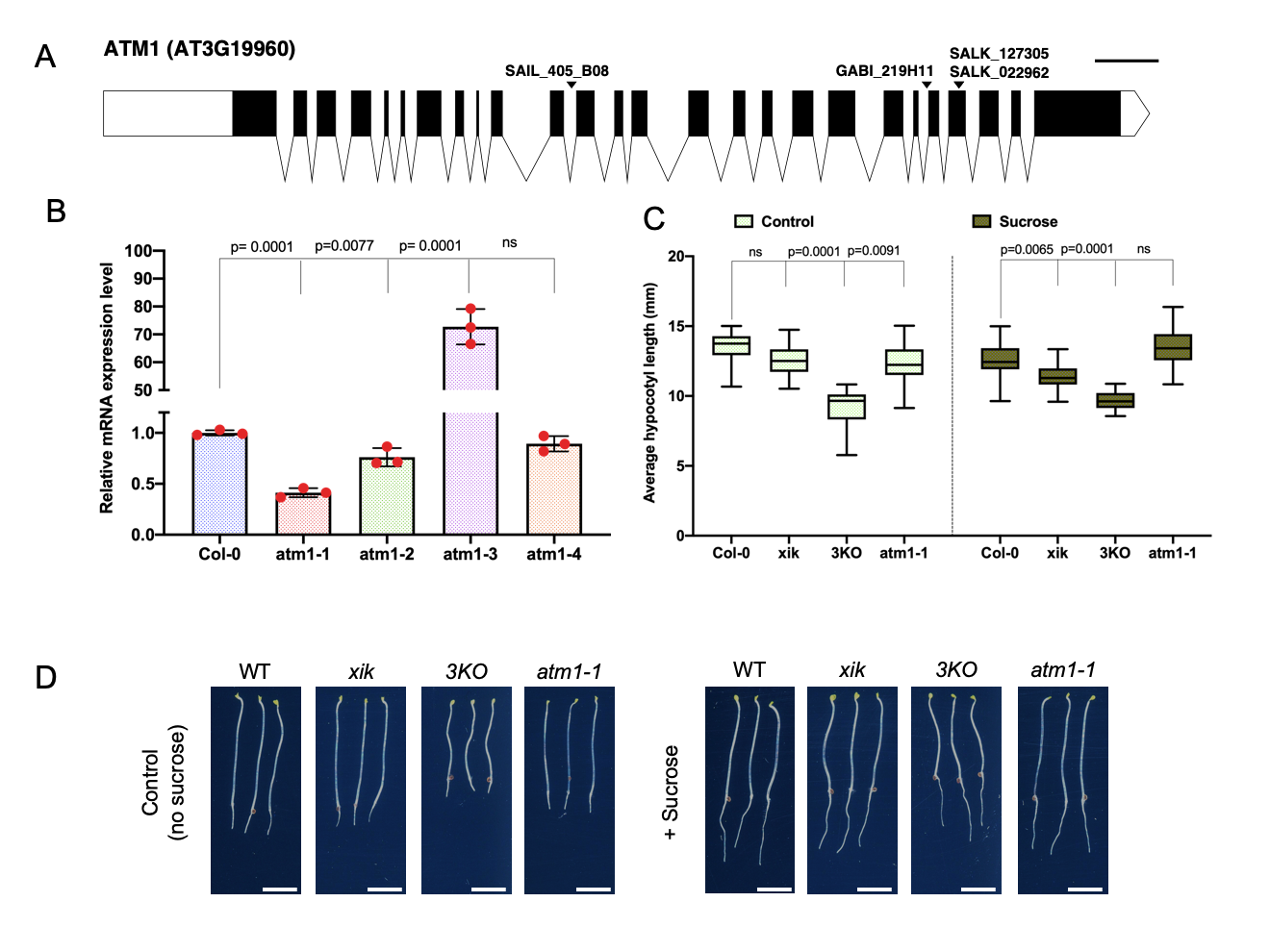
Here, we analyzed the role of plant myosins in sucrose-induced hypocotyl elongation under continuous darkness conditions. To start the molecular characterization of ATM1 (AT3G19960), we analyzed four T-DNA insertional alleles (Fig. 1A), and mutations were confirmed via PCR-based genotyping. We also performed RT-qPCR to quantify ATM1 transcripts in the four alleles, of which atm1-1 (SAIL_405_BO8 previously described by Haraguchi et al., 2014) showed reduced ATM1 expression among these alleles compared to the wild type (Fig. 1B). Notably, atm1-3 exhibits increased mRNA levels and may be a overexpression allele. Since atm1-1 had the lowest ATM1 transcript level, and was previously described as a null (Haraguchi et al., 2014) we decided to use this allele for further phenotypic analysis. To understand the contribution of plant myosins in sugar-regulated hypocotyl cell expansion we performed growth assays using Col-0 (WT: wild-type), atm1-1, a class XI myosin single gene mutant (xik) and the triple knockout line (3KO; xi1 xi2 xik). This triple mutant was previously generated using SALK insertion lines in XI-K (SALK_067972; At5g20490), XI-1 (SALK_019031; At1g17580) and XI-2 (SALK_055785; At5g43900) (Prokhnevsky et. al., 2008; Valera et. al., 2010). All genotypes were grown on 0.5X Murashige and Skoog (MS) medium without or with 15 mM sucrose under constant darkness for 5 days. Under the sucrose-free condition (control), all the three myosin mutants showed reduced hypocotyl length compared to the WT plants (Fig. 1C and D), suggesting the role of myosins in cell elongation. Specifically, the 3KO mutant was severely impaired in hypocotyl cell expansion in the absence of sucrose when compared to the single mutants, hinting that this gene family may redundantly regulate plant development. In the presence of sucrose under constant darkness, the short hypocotyl phenotype was not fully rescued when compared to the WT plants except for atm1-1.
Altogether our findings suggest that plant-specific myosins may be involved in sugar-regulated hypocotyl elongation under continuous darkness. These myosins have diverse subcellular localization patterns (Haraguchi et al., 2014; Peremyslov et al., 2012, 2008) and thus may represent a new downstream signaling output following sucrose signals within the developing shoot. Sucrose-induced hypocotyl elongation has been linked to brassinosteroid, gibberellins, phytochrome interacting factors (PIFs) and SnRK1 (Sucrose Nonfermenting Kinase 1) signaling (Laxmi et al., 2004; Liu et al., 2011; Simon et al., 2018a; Zhang et al., 2015, 2010; Zhang and He, 2015). Other proteins that are important for this process include TANG1 (Zheng et al., 2015), a WD40 protein AtGHS40 (Hsiao et al., 2016), a plant-specific protein SR45 (Carvalho et al., 2010), and HIGH SUGAR RESPONSE 8 (HSR8) (Li et al., 2007). Further studies will be needed to determine how these molecular factors work in concert or parallel to regulate skotomorphogenesis.
Methods
Request a detailed protocolPlant materials
Arabidopsis thaliana plants used in this study were Columbia (Col-0) ecotype: SAIL_405_B08 (atm1-1), SALK_127305C (atm1-2), GABI_219H11(atm1-3) and SALK_022962C (atm1-4) and previously described (xik) and 3KO triple knockout line (xi1 xi2 xik) (Peremyslov et al., 2012, 2010, 2008). Seeds were surfaced sterilized using 50% bleach and 0.01% Triton X-100 for 10 min and then washed five times with sterile water. Seeds were then imbibed in sterile water for 2 days at 4 °C and then transferred to 0.5X Murashige and Skoog (MS) medium plates supplemented without/with 15mM sucrose. For hypocotyl elongation, plates were incubated in the light for 6 hours prior to incubation in the dark for 5 days at 22 °C. PCR based genotyping of the mutants was performed with primers listed in Table 1 and 2X DreamTaq polymerase master mix (Thermo Fisher Scientific).
Real time quantitative PCR (RT-qPCR)
Plant materials were harvested from 7-day old seedlings in three biological replicates. RNA samples were extracted with ZYMO RESARCH Direct-zol RNA Miniprep Plus Kit and quality was spectrophotometrically measured with the Nanodrop. cDNA synthesis was performed with SuperScript IV Reverse Transcriptase kit according to manufacturer’s instruction. The samples were run on BIO RAD CFX Connect Real-Time PCR Detection System with the following components per reaction of 20µL volume: 10µL iTaq Universal SYBR Green Supermix, 0.6 µL Forward Primer F (300nM), 0.6 µL Reverse Primer F (300nM) and 3µL cDNA (5ng/ul). No cDNA samples (water) were included as negative control. Cycling conditions were 5 min at 95 °C, followed by cycles of 15 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C. Data acquisition was done at the end of every cycle. The samples were prepared in three biological and two technical repeats. The Comparative CT Method (ΔΔ CT Method) was used for the analysis of Ct values whereby the amount of target, normalized to an endogenous reference and relative to a calibrator and is given by 2 –ΔΔCT.
Hypocotyl length and statistical analysis
Five-day old dark-grown seedlings were imaged using an Epson Perfection V600 Photo Scanner. Hypocotyl length was measured with ImageJ. For each experimental treatment, 30-40 seedlings were measured for each genotype. The assays were repeated three independent times with similar results. GraphPad Prism (GraphPad Software, version 8.4.2) was used for statistical analysis. To compare treatments effects on the mean value of wild type and mutants, either or one- or two-way ANOVA was performed using Tukey’s test for multiple comparison.
Gene diagram annotation
The diagram of ATM1 (AT3G19960) was created using the Exon-Intron Graphic Maker (http://wormweb.org/exonintron) using current gene model sequence from TAIR (www.arabidopsis.org). The position of all T-DNA alleles examined was obtained from the SALK SIGnAL T-DNA express Arabidopsis Gene Mapping tool (http://signal.salk.edu/cgi-bin/tdnaexpress).
Reagents
Table 1: Primers used in this study.
| Primers | 5′ > 3′ Sequence | Purpose |
| SAIL_405_B08 LP | TTCGTGTGAACGTTGATTCTG | Genotyping |
| SAIL_405_B08 RP | TCCAGCTTGAATAGATGACGG | |
| SALK_127305_LP | TCCTCAAGCATCACCGTTAAC | |
| SALK_127305_RP | GCAGAGAGCTCAAGTGTTTGG | |
| GABI_219H11_LP | TAAGAGCGAGACAGAGAACCG | |
| GABI_219H11_RP | TCGTGGTTGGTTGGTTAGAAG | |
| SALK_022962_LP | GGGGAAACAGAGAGAAATTGG | |
| SALK_022962_RP | TTTGCTTTGGCATTAACCAAC | |
| Sail-LB2 | GCTTCCTATTATATCTTCCCAAATTACCAATACA | |
| LBb1.3 | ATTTTGCCGATTTCGGAAC | |
| GABI-8474 | ATAATAACGCTGCGGACATCTACATTTT | |
| ATM1_qPCR_Fw | CAGACAGAGAACTGAGGAGGC | RT-qPCR |
| ATM1_qPCR_Rv | CATCGAACCACTGCTCTCTTCG | |
| AT1G13320_Fw | GCGGTTGTGGAGAACATGATACG | |
| AT1G13320_Rv | GAACCAAACACAATTCGTTGCTG |
Acknowledgments
We wish to thank Valerian Dolja (Oregon State University) for sharing seed stocks of the previously described (xik) and 3KO triple knockout lines (xi1 xi2 xik) (Peremyslov et al., 2008). All other seed stocks were obtained from the ABRC (Arabidopsis Biological Resource Center at Ohio State University).
References
Funding
This work was supported by an American Association of University Women Research Publication Grant to D.R.K. and Iowa State University start-up funds to D.R.K.
Reviewed By
Michelle Facette and AnonymousHistory
Received: June 11, 2020Revision received: July 3, 2020
Accepted: July 7, 2020
Published: July 10, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Olatunji, D; Kelley, DR (2020). A role for Arabidopsis myosins in sugar-induced hypocotyl elongation. microPublication Biology. 10.17912/micropub.biology.000276.Download: RIS BibTeX