Description
Transient receptor potential (TRP) channels are a family of cation channels that are important for response to diverse external stimuli across eukaryotes (Venkatachalam and Montell 2007; Samanta et al. 2018). In C. elegans, only members of the TRPV family, which includes osm-9, ocr-1, ocr-2, ocr-3 and ocr-4 (Colbert and Bargmann 1995; Colbert et al. 1997; Tobin et al. 2002), have been shown to play a role in chemosensory behavior (Bargmann 2006). OSM-9 and OCR-2 are co-expressed in six pairs of sensory neurons: AWA, ASH, ADL, ADF, PHA and PHB (Colbert et al. 1997; Tobin et al. 2002). In these cells, they are thought to come together to function as a single channel complex to mediate sensory transduction since their localization to the cilia is mutually dependent upon each other (Tobin et al. 2002).
In the literature, OSM-9 and OCR-2 are often referred to as being required for all ASH-mediated avoidance behaviors. Indeed, osm-9 and ocr-2 mutant animals are defective for response to nose touch and high osmolarity, although ocr-2 mutants did retain some ability to respond to the highest osmotic strength tested (4M) (Colbert et al. 1997; Tobin et al. 2002). osm-9 and ocr-2 mutants are also severely defective in avoidance of 2-octanone (Tobin et al. 2002) and 1-octanol (Ezak et al. 2010). Both mutants are defective in avoidance of high pH (Sassa et al. 2013; Wang et al. 2016), and osm-9 mutants are defective in CuSO4 avoidance when the assay was performed in a plate chemotaxis format (Wang et al. 2015). However, osm-9 and ocr-2 single mutants, as well as osm-9 ocr-2 double mutant animals, retain partial response to bitter tastants, including quinine, when tested at 10mM (Hilliard et al. 2004; Ezak et al. 2010). Animals lacking either or both channels also retain an intermediate level of response to the heavy metal copper (10mM CuCl2) and the detergent SDS (0.1%) (Ezak et al. 2010).
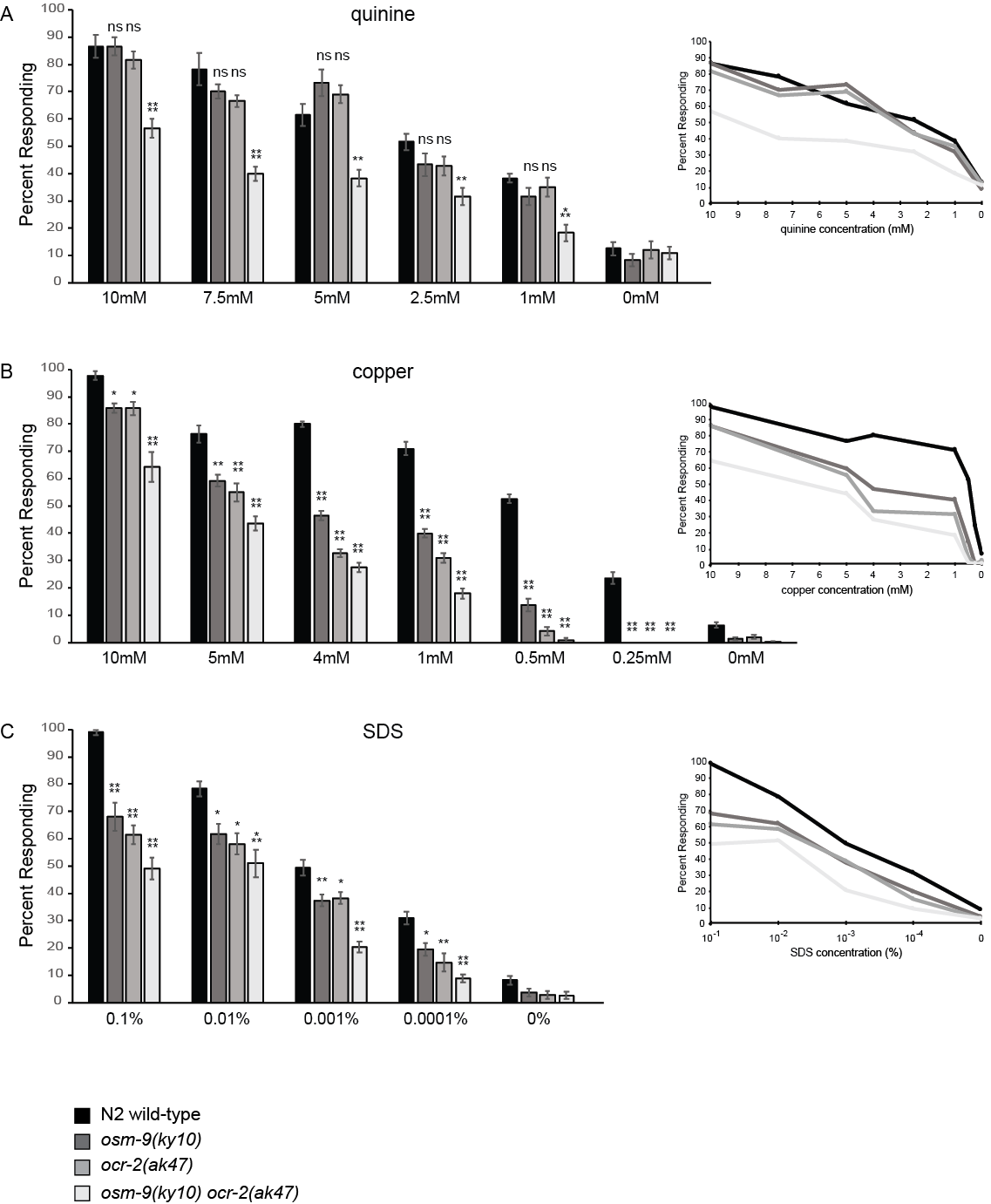
We sought to determine the extent to which OSM-9 and OCR-2 contribute to the ASH-mediated behavioral avoidance of quinine (Hilliard et al. 2004), copper (Sambongi et al. 1999) and SDS (Hilliard et al. 2002). We used the drop assay as previously described (Hilliard et al. 2002; Fukuto et al. 2004; Hilliard et al. 2004; Ezak et al. 2010; Krzyzanowski et al. 2013) to assess the percentage of wild-type, osm-9(ky10), ocr-2(ak47), and osm-9(ky10) ocr-2(ak47) animals responding to each soluble chemical stimulus across a range of concentrations (Figure 1).
Although in one study osm-9 mutant animals were reported to have a modest but statistically significant defect in 10mM quinine avoidance (Hilliard et al. 2004), we found that individual loss of neither OSM-9 nor OCR-2 function affected response to 10mM quinine (Figure 1A), similar to our previous report (Ezak et al. 2010). Further, osm-9 and ocr-2 single mutants responded similarly to wild-type animals across the range of quinine concentrations tested (10mM – 1mM, Figure 1A). Only in the osm-9 ocr-2 double mutant animals was a partial defect in quinine avoidance seen, at each concentration (Figure 1A).
In response to copper (CuCl2), at each concentration the osm-9 and ocr-2 single mutants, as well as the osm-9 ocr-2 double mutant, were partially defective in the avoidance response (Figure 1B). Only at the lowest concentrations tested (0.5mM and 0.25mM) were either the single or double mutant animals essentially lacking response (Figure 1B). Similarly, in response to SDS only a partial avoidance defect was seen for osm-9 and ocr-2 single mutants at 0.1% – 0.001%, with the double mutant showing a somewhat greater defect at 0.001% (Figure 1C).
Taken together, assaying animals at intermediate concentrations of quinine, copper and SDS demonstrated that even when lacking the function of both OSM-9 and OCR-2, a substantial percentage of animals retained behavioral response to these aversive stimuli. Thus, although OSM-9 and OCR-2 are key components in ASH-mediated chemosensory avoidance, their loss leads to diminished, but not absent, avoidance responses at most concentrations tested. This suggests that an additional channel(s) may contribute to chemical avoidance in the absence of these TRPV channels.
Reagents
The strains N2 Bristol wild-type, CX10 osm-9(ky10) and CX4544 ocr-2(ak47) were obtained from the Caenorhabditis Genetics Center, which is funded in part by the National Institutes of Health – Office of Research Infrastructure Programs. LX748 osm-9(ky10) ocr-2(ak47) was a gift from Michael Koelle and has not been sent to the CGC.
Acknowledgments
We thank Michael Koelle for the LX748 strain and Aditi Chaubey for help with statistical analysis.
References
Funding
This work was supported by the National Institutes of Health (grant R01DC015758 to DMF).
Reviewed By
AnonymousHistory
Received: June 22, 2020Revision received: June 27, 2020
Accepted: July 3, 2020
Published: July 8, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Mehle, EA; Sojka, SE; K.C., M; Zel, RM; Reese, SJ; Ferkey, DM (2020). The C. elegans TRPV channel proteins OSM-9 and OCR-2 contribute to aversive chemical sensitivity. microPublication Biology. 10.17912/micropub.biology.000277.Download: RIS BibTeX