Description
The purpose of this study was to determine the utility of the wMicroTracker as a screening platform to assess the motility of various parasites. We tested three species of parasites: the adult and larval stages of the filarial nematode Brugia pahangi, the schistosomula stage of the trematode Schistosoma mansoni, and the epimastigote stage of the protozoan parasite Trypanosoma cruzi. We optimized the assay for the number of parasites per well, plate type and media volume using the wMicroTracker and compared those readouts to readouts from the WormAssay (Marcellino et al. 2012) when possible. The WormAssay has been used in phenotypic drug screens to identify new compounds for the treatment of lymphatic filariasis, onchocerciasis and schistosomiasis (Storey et al. 2014; Bulman et al. 2015; Weeks et al. 2018; Tyagi et al. 2019). The original WormAssay was developed by Marcellino et al. 2012 and was subsequently modified to the “Worminator” by Storey et al. 2014 to observe smaller worms with an inverted microscope. wMicroTracker (InVivo Biosystems) protocols optimized for C. elegans adults (1 mm in length by 80 µm in width, highly motile) were used to optimize parasite assays based on the size and motility of each of the parasite species as compared to C. elegans adults.
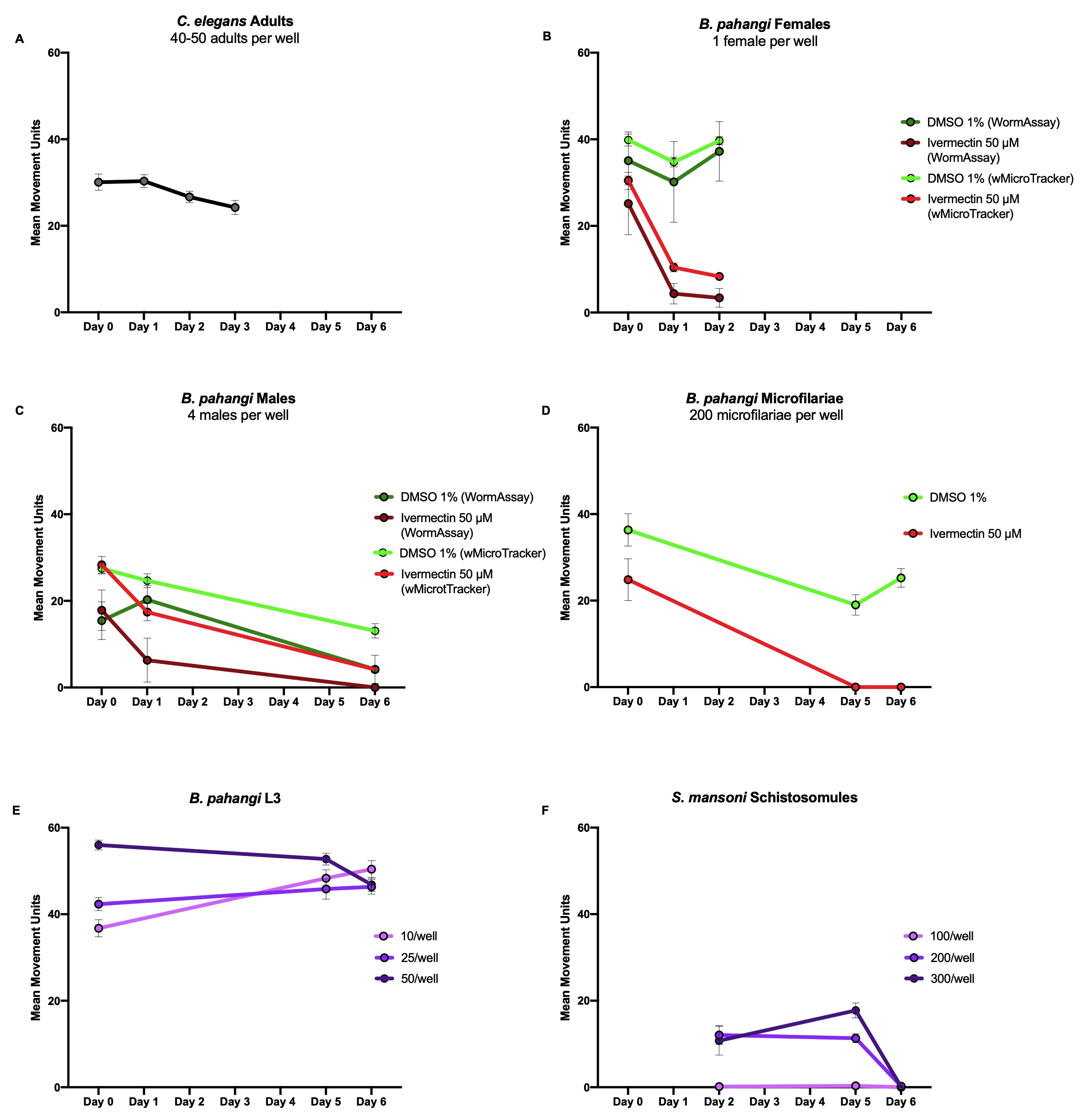
To use the wMicroTracker, two factors need to be considered: the size of the parasite of interest and how active they are. The wMicroTracker detects movement when an organism crosses the stationary LED beam at the center of the well. Hence, parasites that do not travel throughout the well should be assayed in a U-bottom plate to ensure movement is detected. For parasites that do travel throughout the well, a flat bottom plate is sufficient. To determine the number of parasites to use per well, we compared their size to that of C. elegans adults. Following the InVivo Biosystems protocol (https://invivobiosystems.com/wp-content/uploads/2018/12/Protocol_toxicity-in-c-elegans_122018.pdf), C. elegans adults were screened in the wMicroTracker with 40-50 adults per well in 100 µL of M9 buffer (Stiernagle 2006). This produced results in the range of 25-35 mean movement units per well (Figure 1A). To optimize screening methods in the wMicroTracker for other parasites, the size and motility of parasites of interest were compared to C. elegans adults to ensure control wells gave mean movement units around this range.
B. pahangi females (34.7 mm in length by 139 µm in width (Mutafchiev et al. 2014), highly motile) were screened with one worm per well in 500 µL of RPMI in a 24-well flat bottom plate with 8-9 replicate wells. B. pahangi males (18.0 mm in length by 77 µm in width (Mutafchiev et al. 2014), high motility) were screened with four worms per well in 500 µL of RPMI in a 24-well flat bottom plate with 4-7 replicate wells. B. pahangi females and males were assessed in both screening platforms. Both platforms showed similar motility profiles in response to 50 µM ivermectin or 1% DMSO as a negative control (Figure 1B & C).
B. pahangi microfilariae (mf) (177-230 µm in length by 5-7 µm in width (CDC, Lymphatic Filariasis), moderately motile) were screened in the wMicroTracker with 200 mf per well in 100 µL RPMI in both a 96-well U-bottom plate (Figure 1D) and a 96-well half-volume flat-bottom plate (data not shown). Mf were screened with 18 replicate wells per treatment where the positive control was 50 µM ivermectin and the negative control was 1% DMSO. The 96-well half-volume flat-bottom plate was not able to detect mf movement.
B. pahangi third-larval stage (L3) (1-2 mm in length by 26 µm in width (Mutafchiev et al. 2014), highly motile) were screened in the wMicroTracker with 10, 25, and 50 L3 per well in 200 µL RPMI in a 96-well U-bottom plate with 3 replicate wells per condition (Figure 1E). All conditions showed similar motility profiles and produced reliable data.
Schistosoma mansoni schistosomules (110 µm in length by 18 µm in width (Samuelson et al. 1980), not very motile) were screened in the wMicroTracker with 100, 200, and 300 schistosomules per well in 100 µL RPMI in a 96-well U-bottom plate with 8 replicate wells per condition (Figure 1F). Both 200 and 300 schistosomules per well showed generally similar motility profiles and gave reproducible results.
Trypanosoma cruzi epimastigotes (25.6 µm in length by 1.9 µm in width (Gonçalves et al. 2018), moderately motile) were also screened in the wMicroTracker with 10,000, 50,000, and 100,000 epimastigotes per well in 100 µL DMEM in a 96-well U-bottom plate with 6 replicate wells per condition (data not shown). Motility was not detected in the wMicroTracker. Based on these data, it is not known if more than 100,000 parasites per well would allow for the wMicroTracker to detect motility of T. cruzi epimastigotes.
Based on the results of our screens with various parasites using the wMicroTracker and the WormAssay, it is recommended that the WormAssay be used to screen parasites greater than 10 mm in length and that the wMicroTracker and the modified WormAssay, the “Worminator” (Storey et al. 2014), be used to screen parasites less than 1 mm in length. When screening parasites in the wMicroTracker that do not travel throughout the well (e.g. schistosomules, B. pahangi mf, B. pahangi L3) it is recommended to employ a 96-well U-bottom plate to ensure the parasites do not settle at the sides of the well and that they are able to cross the stationary LED beam at the center of the well. When screening parasites that are larger than C. elegans adults in the wMicroTracker, use fewer parasites per well and when screening parasites in the wMicroTracker that are smaller than C. elegans adults, use more parasites per well. The aim is to detect mean movement units around 20-40 per well. We believe that these methods will help the research community develop better screening methods and shorten the time to optimize a screening platform.
Reagents
RPMI: Thermo Fisher Scientific (Catalog #: 22400089)
DMEM: Sigma-Aldrich (Catalog #: D5796)
Ivermectin: Sigma-Aldrich (Catalog #: I8898)
DMSO: Fisher Scientific (Catalog #: BP231-100)
Acknowledgments
The authors would like to acknowledge KC Lim for providing S. mansoni schistosomules, Brenda Beerntsen for providing B. pahangi adults and L3s, and Jeffrey Whitman for providing T. cruzi epimastigotes.
References
Funding
The Bill & Melinda Gates Foundation
Reviewed By
Janis C. WeeksHistory
Received: April 9, 2020Revision received: July 9, 2020
Accepted: July 14, 2020
Published: July 20, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Gunderson, E; Bulman, C; Luo, M; Sakanari, J (2020). In vitro screening methods for parasites: the wMicroTracker & the WormAssay. microPublication Biology. 10.17912/micropub.biology.000279.Download: RIS BibTeX