Description
Lipid metabolism plays an essential role in the survival and adaptation of animals under variable environmental conditions. Lipids are important macromolecules that store energy, serve as structural components, and function as signaling molecules (Watts and Ristow 2017; Papsdorf and Brunet 2019). Defects in lipid metabolism are linked to various diseases and aging in eukaryotes. Therefore, understanding the regulation of this process is critical to modulating disease progression (Wymann and Schneiter 2008).
We have shown earlier that lipid metabolism in C. elegans is regulated by an Axin family member, PRY-1 (Ranawade et al. 2018). While the signaling network of PRY-1 in this process remains to be investigated, Axin family of proteins are known to function in both WNT dependant and independent pathways to regulate various developmental events (Mallick, et al. 2019). Our transcriptomic analysis of both mRNA and miRNA genes revealed that PRY-1 is involved in lipid synthesis by affecting the expression of genes such as fatty acid desaturases (fat-5, fat-6, and fat-7) and vitellogenins (vit-1, vit-2, vit-3, vit-4, vit-5 and vit-6) (Ranawade et al. 2018; Mallick, et al. 2019).
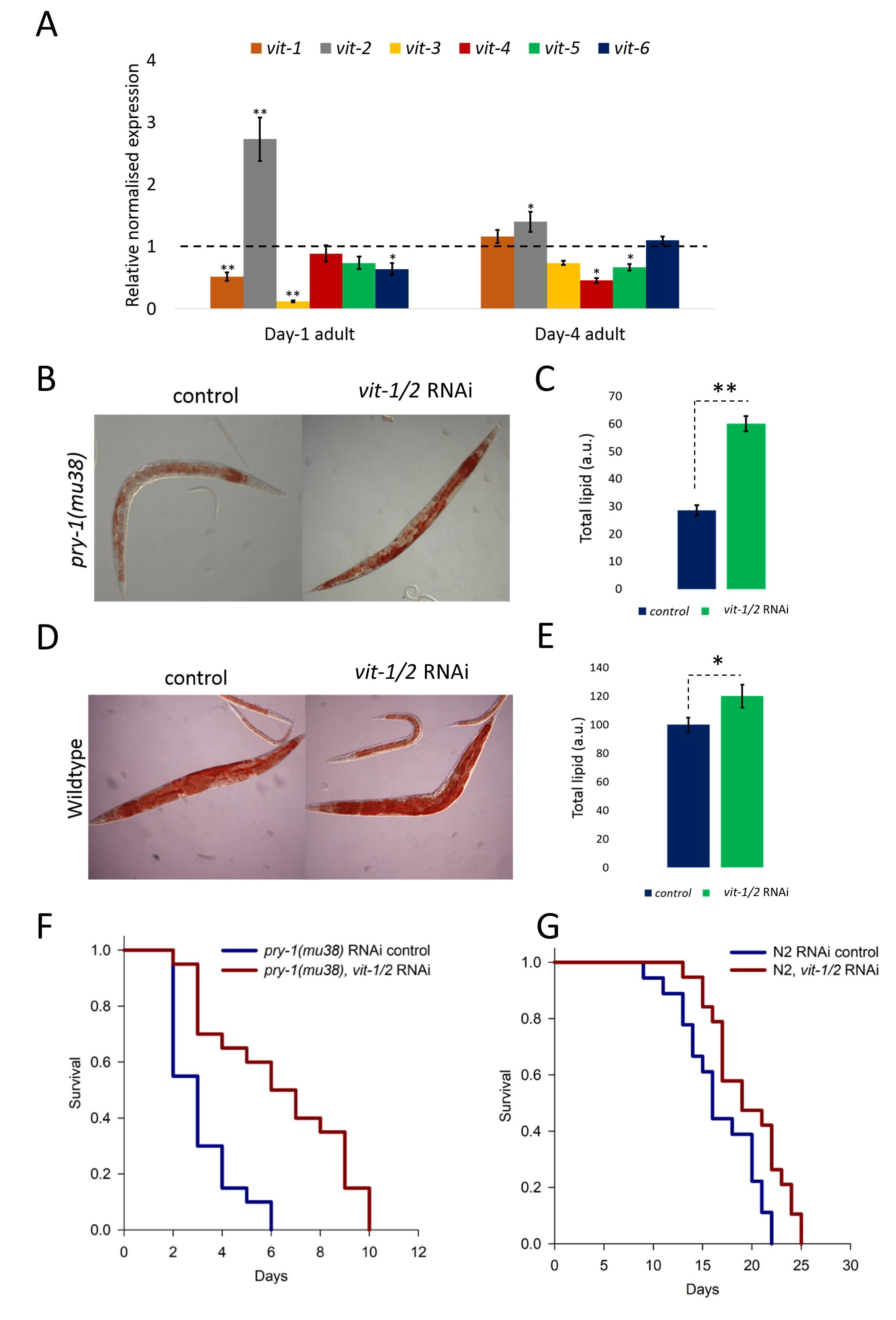
Vitellogenins are yolk lipoproteins, similar to mammalian apolipoprotein B, that bind to complex lipids and aid in their transportation from the intestine to the gonad (Kimble and Sharrock 1983; Grant and Hirsh 1999). Moreover, vit-2 has been shown to negatively regulate longevity and such a role of vit-2 depends on autophagy, lysosomal lipases, DAF-16/FOXO and HLH-30/TFEB (Seah et al. 2016). In this study, we report a new, adult-specific role of vit-2 in pry-1-mediated regulation of lipid levels and lifespan. We analyzed the transcript levels of vit genes in day-1 and day-4 adults and found that vit-2 was the only vitellogenin whose expression was significantly upregulated in pry-1 mutants (Figure 1A). This suggested to us that vit-2 is negatively regulated by pry-1 and may be involved in pry-1-mediated adult-specific processes. To investigate this further, we examined whether vit-2 knockdown during adulthood can rescue the lipid and lifespan defect (Mallick, et al. 2020) of pry-1 mutants. This was done using a vit-1 dsRNA that also knocks down vit-2 due to the sequence similarity (Ranawade et al. 2018). The results showed that the knockdown of vit-1/2 during adulthood significantly rescued lipid levels in pry-1(mu38) (almost 2-fold) (Figures 1B and 1C). Similar experiments in wildtype animals showed a modest increase (by 1.2-fold). We also examined the lifespan phenotype following vit-1/2 RNAi and observed a marked rescue of the lifespan defect (Mallick et al. 2020) in pry-1(mu38) (102% increase in mean lifespan). The wildtype animals showed a comparatively lower increase in the mean lifespan (16.6%) (Figures 1F and 1G). Overall, these findings show that vit-2 functions downstream of pry-1 to regulate both lipid levels and lifespan.
Methods
Request a detailed protocolStrain and growth conditions
Worms were grown at 20°C on standard nematode growth media plates seeded with E. coli OP50. The strains are N2 (wildtype C. elegans) and DY220 pry-1(mu38) I.
Lifespan analysis
Lifespan experiments were performed as described previously (Murphy et al. 2003) at 20oC. All experiments were performed on RNAi plates with HT115 cells expressing either empty vector (L4440) or dsRNA of vit-1/2 gene (The Ahringer C. elegans RNAi feeding library, sjj_K09F5.2, location X-4A17, FP: CATGCTTGCTTTGTGGAGAA and RP: TTTGAGAATCCTGGGAAACG). Synchronized animals were transferred onto RNAi plates at L4 stage and observed every day throughout the lifespan.
Oil Red O staining
Oil Red O staining was performed as previously reported (Ranawade et al. 2018). Animals at day-1 adulthood were collected after washing with 1X PBS buffer from the plate and treated as described in the protocol. Animals were mounted and imaged with a Q imaging software and Micropublisher 3.3 RTV color camera outfitted with DIC optics on a Nikon 80i microscope. NIH ImageJ software was used to quantify Oil Red O intensities (Soukas et al. 2009). 15 to 30 worms were randomly selected from each category in at least two separate batches.
qPCR analysis
Total RNA was extracted from bleach synchronized worms by Tri-reagent (Catalog Number T9424, Sigma-Aldrich Canada) according to the manufacturer’s instructions. Using oligo (dT) primers cDNA was made from total RNA with SensiFAST™ cDNA kit (Catalog Number BIO-65054, USA). Quantitative real-time PCR (qRT-PCR) analysis was performed on a CFX 96 BioRad cycler in triplicate with SensiFAST™ SYBR® Green Kit (Catalog Number BIO-98005, USA), according to the manufacturer’s instructions. Primers used in the study are listed below: pmp-3 (FP: CTTAGAGTCAAGGGTCGCAGTGGAG and RP: ACTGTATCGGCACCAAGGAAACTGG), vit-1 (FP: GGTTCGCTTTGACGGATACAC and RP: AACTCGTTGGTGGACTCATC), vit-2 (FP: GACACCGAGCTCATCCGCCCA and RP: TTCCTTCTCTCCATTGACCT), vit-3 (FP: GGCTCGTGAGCAAACTGTTG and RP: TTAATAGGCAACGCAGGCGG), vit-4 (FP: TGTCAACGGACAAGAGGTTG and RP: TCCTTTGGTCCAGAGACCTTC), vit-5 (FP: GGCAATTTGTTAAGCCACAA and RP: CCTCCTTTGGTCCAGAAACCT) and vit-6 (FP: AGTCGCTATTGTCGAGCGTC and RP: AGACGGAGGTCACCTTTTGC).
Statistical analysis
For lifespan analysis, all statistics were performed using SigmaPlot software 11. Survival curves were estimated using the Kaplan-Meier test, and differences among groups were assessed using the log-rank test. Survival data are expressed relative to the control group. Other statistics were performed using Microsoft Office Excel 365. Bio-Rad CFX manager was used for Ct and p values of qPCR analysis.
Acknowledgments
N2 strain was provided by Caenorhabditis Genetics Centre (CGC).
References
Funding
This work was supported by NSERC Discovery grant to Bhagwati Gupta.
Reviewed By
AnonymousHistory
Received: July 6, 2020Revision received: July 17, 2020
Accepted: July 17, 2020
Published: July 21, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Mallick, A; Gupta, BP (2020). Vitellogenin-2 acts downstream of PRY-1/Axin to regulate lipids and lifespan in C. elegans. microPublication Biology. 10.17912/micropub.biology.000281.Download: RIS BibTeX