Fly Facility, National Centre for Biological Sciences (NCBS), TIFR, Bangalore 560065 INDIA
Description
Conjugation of Small ubiquitin-like modifier (SUMO) to a protein substrate (also called SUMOylation) is achieved by a specialized cellular machinery that includes a SUMO maturing enzyme (Ulp1, Ubiquitin-like protease 1) which also doubles as a de-conjugase, heterodimeric activation enzymes (Aos1 & Uba2), a single E2 conjugase (Ubc9), and multiple E3 ligases (Hay, 2005). Loss of function ubc9 mutants shows activated immune signaling (Chiu et al., 2005; Paddibhatla et al., 2010), with Toll signaling attenuated by SUMO conjugation (Bhaskar et al., 2002; Chiu et al., 2005; Paddibhatla et al., 2010). Although multiple studies have confirmed the role of SUMOylation in the regulation of Drosophila innate immune signaling (Bhaskar et al., 2000); Bhaskar et al., 2002; Chiu et al., 2005), only a handful of proteins have been identified as direct targets of SUMOylation. Validated direct targets include Dorsal (Bhaskar et al., 2002), and IRD5 (Fukuyama et al., 2013), critical proteins in the Toll/NFkB and IMD/NFkB cascades, respectively. Previously, to gain a more elaborate understanding of the SUMO conjugation in the immune response, we performed a quantitative proteomic analysis and identified proteins whose SUMOylation status is altered in response to crude LPS, which activates both Toll/NFkB and IMD/NFkB signaling in Drosophila macrophage-like S2 cells. This analysis identified ~700 SUMO targets of which 5% were known to have immune function (Handu et al., 2015).
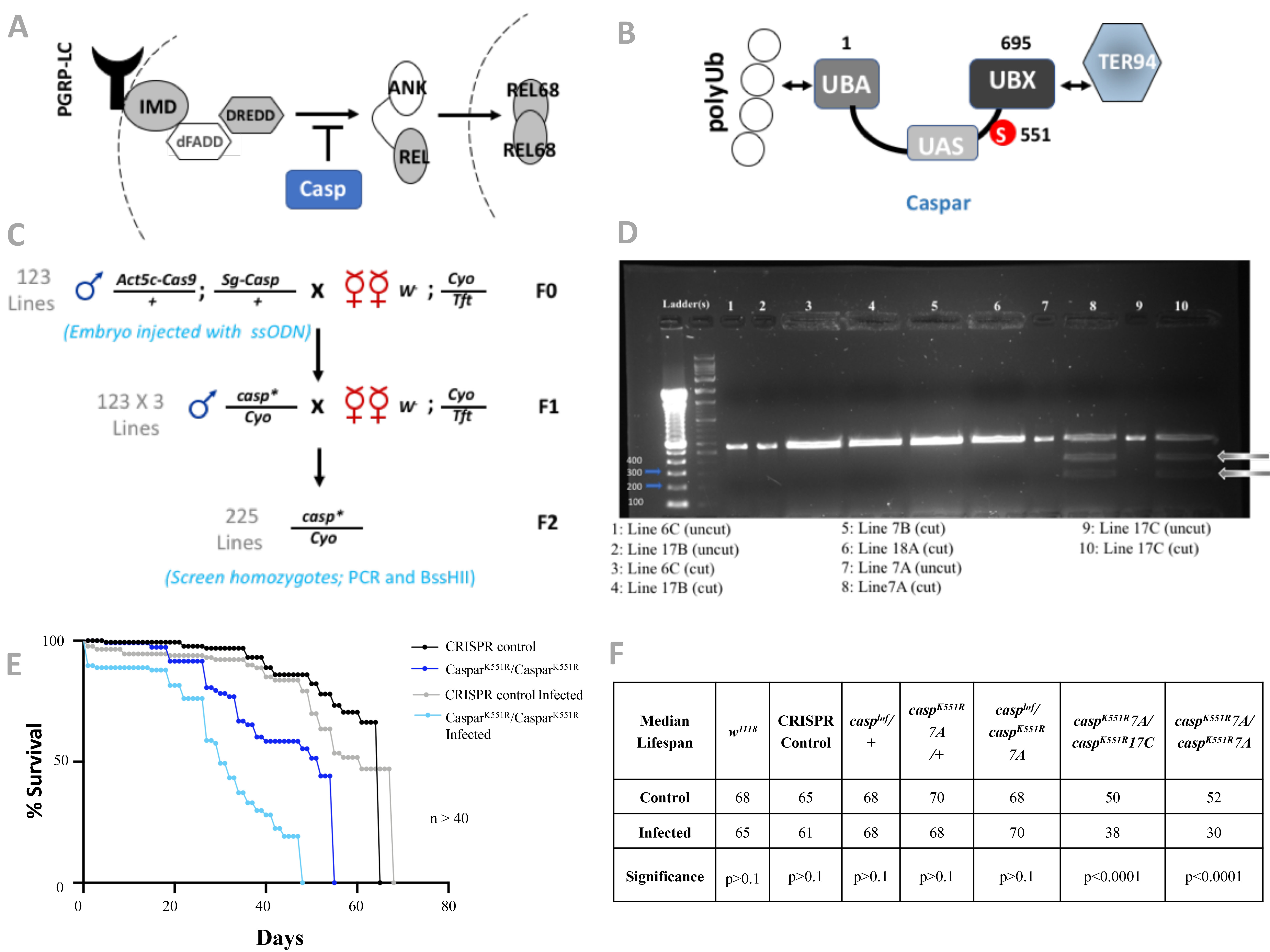
In Drosophila, the IMD/NFkB pathway (Fig. 1A) is activated primarily in response to gram-negative bacterial infections (Brennan & Anderson, 2004; Kaneko & Silverman, 2005; Lemaitre et al., 1995). When the PGPRP receptors recognize a pathogenic signature, a series of activation events leads to DREDD-dependent cleavage of the NFkB transcription factor Relish (Rel). This leads to translocation of cleaved Rel (Rel68) from the cytoplasm to nucleus and expression of numerous defense genes (Stöven et al., 2000). All immune signaling pathways need strong negative regulation in the absence of infection as chronic expression of defense genes is detrimental to the health of the organism. One such negative regulator, for IMD signaling, is Caspar (Casp) (Fig1A, B). Casp negatively regulates the cleavage of Rel, by regulating DREDD activity, and thus retains the transcription factor in the cytoplasm (Kim et al., 2006). Casp is a SUMO target that changes its SUMOylation state with infection (Handu et. al., 2015). Using an in bacto SUMOylation assay (Nie et al., 2009), we have earlier established that Casp is SUMOylated at K551 (Handu et. al., 2015). Based on the domain structure of Casp (Fig. 1B), it possibly functions as an adapter for the ER-associated degradation pathway, assisting in the targeting of unfolded proteins to the ubiquitin-proteasomal system, using its N and C terminal domains to connect ubiquitinated proteins to TER94 (Tresse et al., 2010).
To better understand the SUMOylation-specific biological effects associated with a protein, it is imperative to study it when its endogenous expression patterns and concentrations are maintained. To achieve this, we used CRISPR-Cas9 based genome editing to generate caspK551R transgenic lines. CRISPR mutagenesis techniques involve injecting a mixture of casp guide-RNA (gRNA) and donor DNA (ssODN, single-stranded oligonucleotide with codon CGC instead of AAG) into Cas9-expressing transgenic flies. The sequences of the gRNA and ssODN used are as listed in Materials & Methods. We initially utilized this ‘direct-injection’ protocol on 500 embryos but failed to obtain mutants. One reason for the failure could be the low efficiency of recognition of the casp genomic sequences by the designed gRNA. A DRSC tool (https://fgr.hms.harvard.edu/crispr-efficiency) predicts a score of 5.8 for this gRNA, much lower than the recommended 7 or above for successful editing. For a ssODN based strategy to generate a site-directed mutant, the gRNA sequences chosen had to be at/near the site of mutation for efficient homologous recombination, which limited our ability to design an alternate gRNA. Hence, keeping the gRNA constant, we decide to increase efficiency by using the alternate but slower protocol of first generating a U6.2-casp-gRNA transgenic line based on the protocols of (Kondo & Ueda, 2013). Subsequently, we crossed the U6.2-casp-gRNA transgenic flies with Cas9-expressing flies and collected the F0 embryos. We injected the ssODN containing the K551R mutation in ~350 embryos. Only a small fraction of the adults that eclosed from these injected embryos (Fig 1C) were expected to have gained the mutation. We chose only male flies (n=123) and crossed each male to five w1118; Cyo/Tft females. In the F1 generation, from each numbered vial we took three independent males (e.g Labeled 1A, 1B, 1C for the first line) and generated a 123 X 3 = 369 stocks for screening. With no known visible phenotype, efficient screening of flies with the desired mutation was facilitated by the addition of a unique restriction site—BssHII—at the site of mutation during ssODN design. The genomic DNA flanking the site of mutation was PCR amplified and digested with BssHII to check for the presence of the mutation (Fig.1D). We found that detection of the BssHII cleavage site in PCR amplified genomic fragments was easier to score in homozygous flies than in balanced heterozygotes. After screening 225 individual lines, two independent caspK551R mutant lines were obtained, namely lines 7A and 17C (Fig. 1D). The two lines are homozygous viable, have no obvious morphological defects. The lines have the desired codon substitution and no additional mutations/lesions in the casp locus. Quantitative real-time PCR confirmed comparable mRNA expression for wild type (DCt = 5.087) and the generated mutants (DCt = 4.343).
The lifespan of the 7A and 17C lines was measured (Fig. 1E, F) and we found that at 25 °C, both the caspK551R/caspK551R lines had shorter lifespans than the controls (w1118 or caspK551R/+ or CRISPR control), with the change in median lifespan being shorter by 13-20 days, suggesting that Casp SUMOylation contributed to a normal lifespan. A previous study has shown that overexpression of Casp reduces the lifespan of flies post-infection (Kim et al., 2006) linking the activity of Casp to lifespan. Further, Escherichia coli infection shortened the lifespan of the caspK551R/caspK551R by an additional 12 days (Fig. 1E, F) with controls (w1118 or caspK551R/+ or CRISPR control), being able to deal with the E. coli infection without significant change in median lifespan. Interestingly a the caspK551R/casplof line behaved like control with a median lifespan of 70 days without and 68 days with an infection. In our hands, the casplof line shows low levels (~5-10%) of Caspwt expression when compared to w1118.
To the best of our knowledge, this is the first study involving the application of CRISPR mutagenesis to generate a protein variant that is SUMO conjugation resistant. It is not clear at this point if CaspK551R is a stronger or weaker negative regulator of DREDD mediated Rel cleavage. If the mutant protein is a better negative regulator, it may lead to a slower and/or weaker IMD/NFkB immune response. If the allele is weaker negative regulator, it may lead to overactive IMD/NFkB signaling and subsequent inflammation. Either of the above possibilities would lead to a shorter lifespan and increased susceptibility to gram-negative bacteria. This exact mechanism remains to be uncovered.
All the data taken together suggest a need of a delicate balance between the number of non-SUMOylated and SUMOylated Casp moieties for a regulated immune response. FAF1, the mammalian homolog of Casp, also regulates NFkB signaling by regulating the levels and nuclear translocation of RelA (Menges et al., 2009). Although FAF1 itself has not been demonstrated to be SUMOylated, it has SIM motifs and interacts with SUMOylated proteins indicating a critical role of SUMOylation in FAF1 regulated processes (Wang et al., 2019). It will be worth investigating whether molecular mechanisms for both Casp and FAF1 in immune signaling are conserved by the evolutionary process.
Methods
Request a detailed protocolFly strains and maintenance: All flies were maintained on standard cornmeal media at 25 °C. The casplof line (11373) is a piggyBac insert in the casp locus and was procured from the Bloomington Drosophila Stock Centre (BDSC).
Survival Analysis. For survival assays, 2-day old males from each genotype were maintained on standard medium at 25 °C. For survival post infection, 100 flies were pricked with a 20-hour old culture of ampicillin-resistant E. coli (DH5α). Dead flies were removed every day and food vials were changed every alternate day. Surviving flies were scored till all the flies were dead. Gehan-Breslow-Wilcoxon and Log Rank tests were performed using GraphPad Prism 8.0 to analyze the data (Piper & Partridge, 2016).
CRISPR cloning and mutagenesis: Sg2-Casp gRNA, TGATCAGGTGAAGGCAGAGCAGG cloned in pBFv-U6.2B. This clone was injected in y1, v1, phiC1 integrase; attp40 embryos and the progeny were scored for v+ marker. The integrase was removed and the transgenics lines made homozygous.
ssODN sequence:
TTGCCGCATCCTTAGCCATGTCGGCTTGCAAAGTTTCCTGATAGGCCATGTCCTGCTCTGCGCG CACCTGATCACGGGCAGCGCGTTCGTCCTCTTGCCG
Screening and sequencing of mutants: Genomic DNA was extracted from individual homozygous flies, one from each line. PCR primers, casp-F CTGGGCAGCAATTGCGAAGT and casp-R: GTAGCACCTACCTGTAGGTTG were used to amplify a 566 bp PCR product (Figure 1D). PCR products from lines that showed BssH1 digestion, as well as controls that did not show digestion were sent for sequencing. Lines 7A and 17C were confirmed to have the AAG to CGC mutation, while the control lines retained the wild-type codon (AAG). Next, the entire casp locus for lines 7A and 17C was sequenced and was found to be identical to controls, with the exception of the engineered CGC mutation. The engineered mutation has remained stable in both 17C and 7A lines to date (~1 .5 years). casp expression levels were tested using quantitative real-time PCR with primers, casp F- CAAGCGTTTCCAATGTCAGAG; casp R – CTTAGAGCCTCCACAAGATTCC in the 7A, 17C lines as well as CRISPR controls.
References
Funding
Science and Engineering Board (SERB), Govt. of India grant CRG/2018/001218 to DT and GR. Department of Biotechnology (DBT) BT/PR26095/GET/119/199/2017 Govt. of India grant to GR.
Reviewed By
Anonymous and Steven MarygoldHistory
Received: May 15, 2020Revision received: July 23, 2020
Accepted: July 23, 2020
Published: August 2, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Kaduskar, B; Trivedi, D; Ratnaparkhi, GS (2020). Caspar SUMOylation regulates Drosophila lifespan. microPublication Biology. 10.17912/micropub.biology.000288.Download: RIS BibTeX