Description
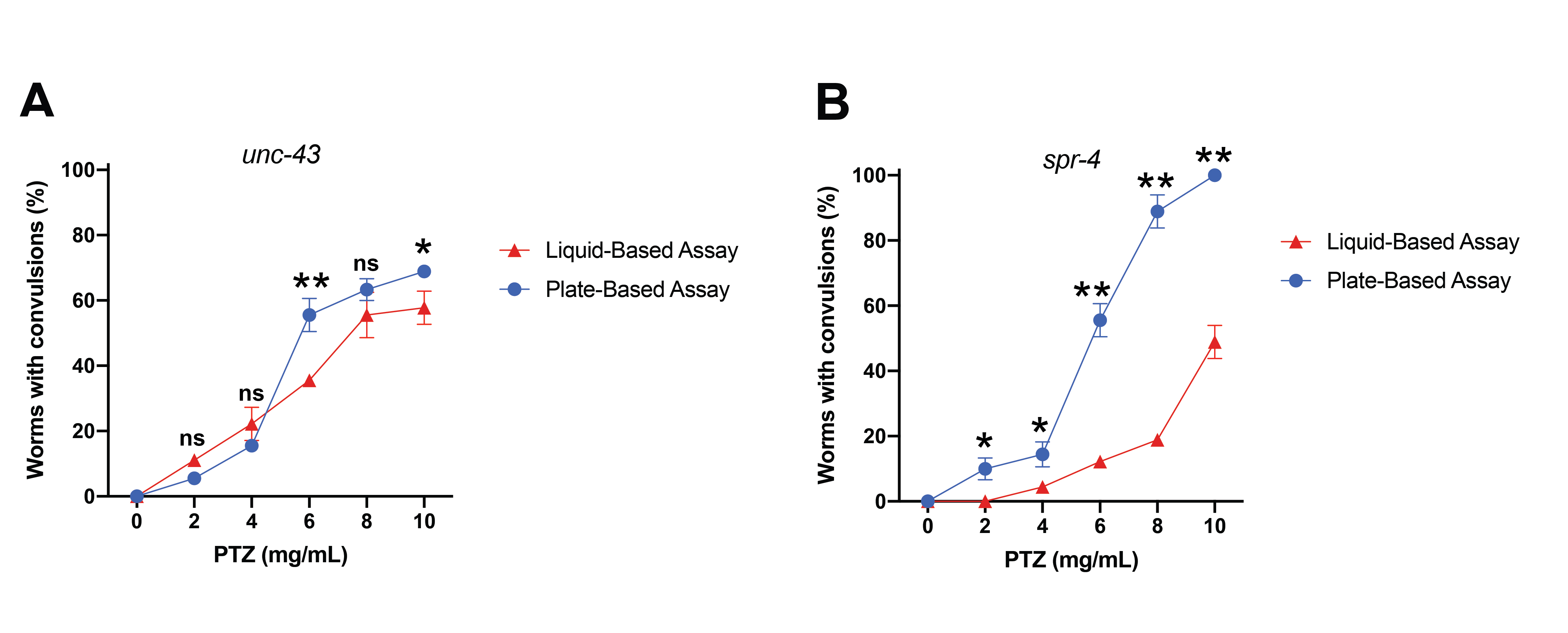
The restrictive element-1 silencing transcription factor (REST)/neuron-restrictive silencing factor (NRSF) is a transcriptional repressor which actively suppresses genes involved in a wide array of neuronal processes such as global ischemia, neurodegenerative conditions and seizure disorders (Calderone et al., 2003; Lu et al., 2014; McClelland et al., 2011). In C. elegans, spr-4 is the homolog of REST; a mutation in spr-4(by105) enhanced amyloid-beta-induced neurotoxicity of glutamatergic neurons in a worm Alzheimer’s disease model (Lu et al., 2014). Previously published research in mammals has reported that REST contributes to the onset of epilepsy via repression of specific genes (McClelland et al., 2011). Additionally, there is a 2- to 6-fold increase in the prevalence and incidence of seizures in Alzheimer’s disease patients (Nicastro et al., 2016). Taken together, we hypothesized there might be a REST-dependent convulsion phenotype in spr-4(by105) mutants. We previously developed a protocol for studying epileptic-like convulsions in C. elegans that involved treating worms with the GABA antagonist, pentylenetetrazole (PTZ) on agar plates (Locke et al., 2008). Here, we tested spr-4(by105) worms for convulsions using our previously developed agar plate assay and a newly developed liquid-based assay from another lab (Wong et al., 2018), both of which used PTZ to induce convulsions. As a negative control, we examined N2 wild-type animals. It should be noted that these wild-type animals did not exhibit a single convulsion at any concentration (2-10 mg/mL PTZ) with either the liquid or plate-based assay. For both assays, we used the unc-43(n498 n1186) worms as a positive convulsion control (Fig. 1A) as these animals are responsive to PTZ on agar plates (Williams et al., 2004) and in liquid containing PTZ (Wong et al., 2018). Notably, the behavioral response of the unc-43 animals was similar in both assay types. The unc-43 animals had more active phenotypes at higher concentrations (6, 8, and 10 mg/mL PTZ) where they displayed a “rubberband”, or clonic convulsion, phenotype. At lower concentrations (4 mg/mL) the animals exhibited either a rubberband or paralysis phenotype. Paralyzed animals still displayed pharyngeal activity and completely recovered normal movement within 20-30 minutes following removal from PTZ. These control animals informed our experimental conditions. We wanted to determine if the convulsion response in spr-4 mutant worms would be similar between the assays or preferentially modulated. Notably, populations of spr-4 mutant animals displayed significantly different convulsion levels at every concentration of PTZ in response to these alternate assay conditions (Fig. 1B). In all cases, the population of animals responsive to PTZ was significantly lower using the liquid-based assay in comparison to the plate-based assay. With both assays, however, the spr-4 worms did display similar types of phenotypic convulsions at comparable concentrations. Specifically, at higher concentrations of PTZ (8 and 10 mg/mL) spr-4 worms exhibited “tonic-clonic”, or head-bobbing, convulsions where the anterior region of the animal had a repetitive movement while the posterior was paralyzed. Conversely, at lower concentrations of PTZ (2 and 4 mg/mL), spr-4 animals displayed primarily full body paralysis. We do not know why spr-4 animals displayed such stark differences in convulsion levels following exposure to PTZ from these two assay types when such distinct and contrasting variability was not displayed by the unc-43 worms. However, it is known that environmental circumstances can induce convulsions in humans. In this regard, we suggest that in our worm assays, mechanosensory or osmotic stress differences might modulate the PTZ-induced epileptic-like convulsive behavior of certain C. elegans mutants in an assay and concentration-dependent manner.
Methods
Request a detailed protocolPlate-based assay. 60 mm NGM agar plates were prepared 48 hours before the assay and stored in a 20oC incubator. A PTZ stock of 0.5 g/mL (Sigma) in ddH2O was prepared fresh on the day of analysis. Appropriate concentrations of PTZ (2-10 mg/mL) were applied to the tops of the NGM plates with a cell spreader/hockey stick. PTZ was dissolved in water; thus, solvent-only (0 mg/mL) plates were also prepared as controls. The plates were dried for 60 minutes with the lids ajar in a sterile hood. The PTZ plates were then seeded with E. coli OP50, which was dried for 30 minutes, in a sterile hood, with the lids ajar, before use. Three plates per concentration/strain were prepared, as each mutant was examined in triplicate. For each mutant, 30 animals were then transferred/plate and examined for convulsion activity (Locke et al., 2008). As worms exhibited convulsion phenotypes, they were removed from the plate and the type of convulsion phenotype and time of the convulsion was noted.
Liquid-based assay. Liquid for C. elegans immersion consisted of Dent’s Ringer solution (DRS). Thirty day 3, young adult worms per experimental condition were placed in 50 μL liquid droplets of different concentrations of PTZ (2-10 mg/mL) dissolved in DRS containing 0.1% bovine serum albumin placed on plastic Petri dish lids. The worms were incubated in this solution for 30 minutes. Following the incubation period, the worms were transferred from the droplet with an eyelash pick and placed on to an NGM plate seeded with 25 μL of E. coli OP50. Worms were then observed for convulsive phenotypes for 1 minute (Wong et al., 2018). As behavioral phenotypes were identified, worms were removed. Each strain was examined in triplicate, with 30 animals examined/replicate, for a total of 90 animals.
Statistics. For either assay, statistical analysis was performed with a Two-Way ANOVA with a Sidak’s post-hoc analysis (GraphPad).
Reagents
C. elegans strain MT2605, unc-43(n498 n1186)
C. elegans strain LA95, spr-4(by105)
E. coli strain OP50 (saturated culture, previously grown in LB and stored at 4°C)
Pentylenetetrazole (Sigma)
Cell spreader/hockey stick (Thomas Scientific, 7012Q52)
Dent’s Ringer solution (DRS), 140 mM NaCl, 1 mM MgCl2, 3 mM CaCl2, 6 mM KCl, 10 mM HEPES, pH 7.4.
References
Funding
An Undergraduate Creativity and Research Academy grant from The University of Alabama College of Arts & Sciences was awarded to Madeline Vaji for this research.
Reviewed By
Brent NeumannHistory
Received: July 20, 2020Revision received: August 19, 2020
Accepted: August 20, 2020
Published: August 20, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Vaji, MA; Caldwell, GA; Caldwell, KA (2020). Phenotypic modulation of pentylenetetrazole-induced convulsive behaviors in C. elegans carrying a mutation associated with Alzheimer’s disease. microPublication Biology. 10.17912/micropub.biology.000295.Download: RIS BibTeX