Description
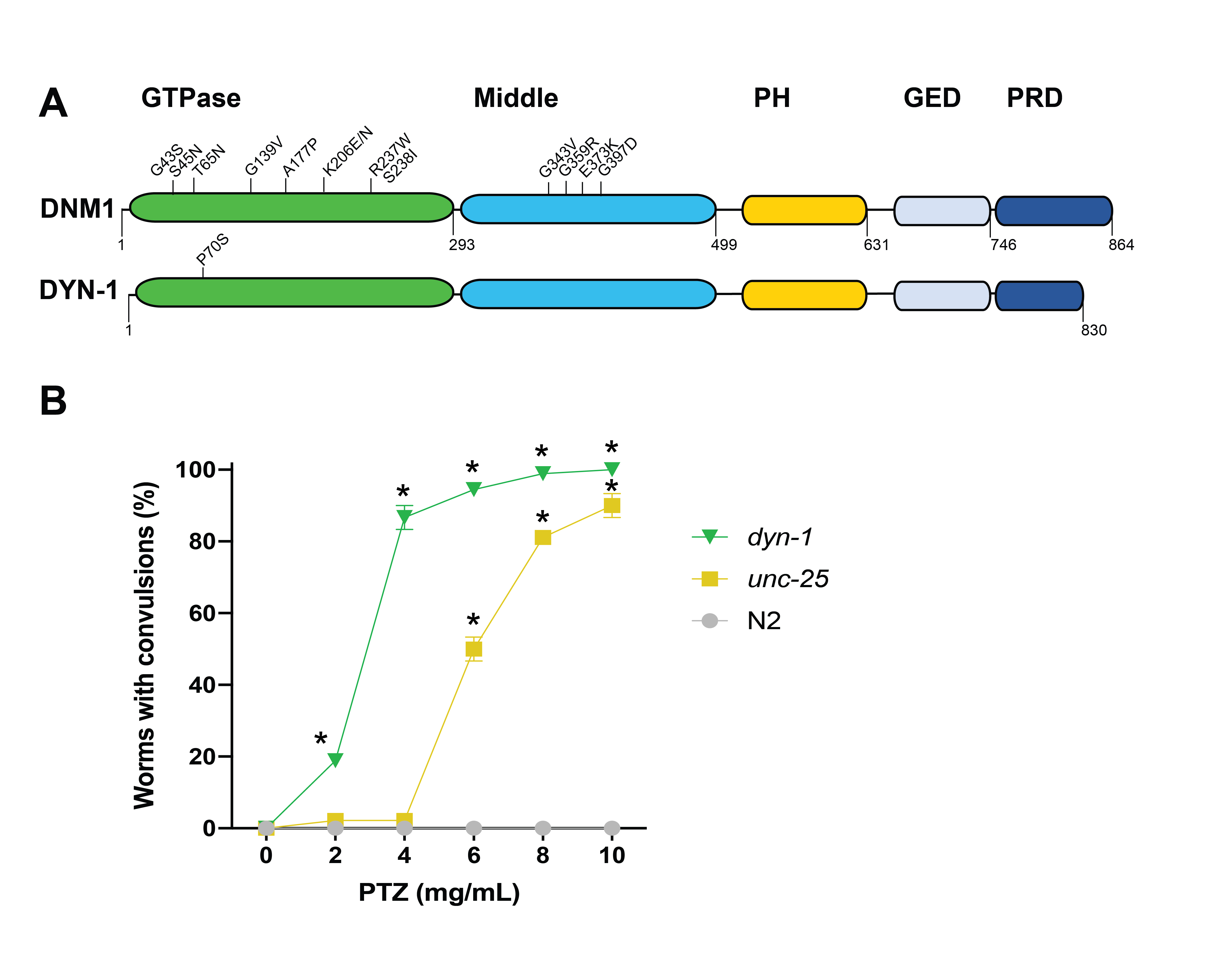
DNM1 encephalopathy in humans is a neurological disorder that causes severe epilepsy with mutations in the gene DNM1 (dynamin 1). This syndrome begins in childhood and persists through adulthood, requiring life-long management of severe seizures. The underlying cause of the seizures is not well understood. However, missense mutations associated with DNM1 encephalopathy are clustered within either the GTPase domain or the middle domain, which, along with the GTPase effector domain (GED), is required for oligomerization and stimulation of GTPase activity (Fig. 1A) (Von Spiczak et al., 2017). The nematode, Caenorhabditis elegans, is a proven model organism for studying epilepsy due to the ability to induce epileptic-like convulsions when mutant animals are treated with pentylenetetrazole (PTZ) (Williams et al., 2004). C. elegans possess a homolog of human DNM1, termed dyn-1. Notably, there is a mutant, dyn-1(ky51), with a missense substitution (P70S) in the GTPase domain (Fig. 1A) (Clark et al., 1997). The nematode proline residue is conserved in human DNM1 (P68). Through detailed analyses in multiple organisms, dynamin 1 has been characterized to function in endocytosis and synaptic transmission (Ferguson and De Camilli, 2012). In this context, we hypothesized that we could induce epileptic-like convulsions in C. elegans dyn-1(ky51) animals following treatment with the drug PTZ. Accordingly, we observed a dose-dependent convulsive effect in dyn-1(ky51) animals (Fig. 1B). These convulsions were similar to the positive control unc-25(e156). UNC-25 is an ortholog of human glutamate decarboxylase and is involved in GABAergic synaptic transmission. The dyn-1 animals exhibit a singular phenotype following exposure to PTZ; they displayed posterior paralysis with repetitive anterior convulsions at all tested concentrations, a phenotype we termed “head bobbing” that we associate with a tonic-clonic convulsion phenotype (Williams et al., 2004). Furthermore, the dyn-1 animals displayed the tonic-clonic phenotype almost immediately upon exposure to PTZ. The unc-25 worms, however, displayed two different convulsion phenotypes following PTZ exposure. When exposed to 8 and 10 mg/mL PTZ, these animals also displayed head bobbing (tonic-clonic) convulsions and they occurred fairly quickly upon exposure to the drug. However, at a lower concentration, 6 mg/mL of PTZ, the phenotype of unc-25 worms was a mixture of both heading bobbing (tonic-clonic) and full body paralysis (tonic convulsions) and it took longer for these phenotypes to appear (40 minutes on average); in this scenario the animal is stiff and will not respond to stimulus, but is still viable, as evidenced by pharyngeal activity. In summary, while dyn-1(ky51) animals displayed a consistent and immediate convulsion phenotype regardless of the concentration, phenotypes for unc-25(e156) worms were dependent on PTZ exposure concentration. It cannot be ruled out that the convulsive differences observed between these strains arise because different temperatures were used to raise the dyn-1 animals (16°C) vs. the unc-25 (20oC) animals.
Methods
Request a detailed protocolThe dyn-1(ky51) mutation is a temperature-sensitive missense mutation that expresses wild-type dynamin at temperatures lower than 16°C, but within 30 minutes of exposure to 25°C, misfolded and non-functional protein is produced (Clark et al., 1997). When animals reached the L4 stage, they were shifted from the permissive temperature (16°C) to the restrictive temperature (25°C) and examined for convulsions on PTZ-containing Petri dishes. N2 and unc-25 worms were grown at 20°C until they also reached the L4 stage and were exposed to PTZ-containing plates.
NGM agar plates were made 48 hours before use and stored in a 20oC incubator. On the day of analysis, concentrations of 2-10 mg/mL PTZ (Sigma) were spread on top of the NGM plates using a cell spreader/hockey stick and dried for 60 minutes in a sterile hood with the lids ajar; solvent plates containing 0 mg/mL PTZ as “water only” controls were also prepared. Since the experiments were performed in triplicate, three plates per concentration/strain were utilized. The PTZ plates were then seeded with E. coli OP50, placed in the center of each plate, which was then allowed to dry for 30 minutes, with the lids ajar, in a sterile hood.
For each strain, 30 animals were then transferred to each plate and examined for convulsion activity for up to 60 minutes. When a worm displayed a convulsion, the phenotype was noted and the worm removed from the plate. Each strain was examined in triplicate, with 30 animals examined on each plate, for a total of 90 animals. Statistical analysis was performed with a Two-Way ANOVA with a Dunnett’s post-hoc analysis (GraphPad).
Reagents
C. elegans strain CB156 unc-25(e156)
C. elegans strain CX51 dyn-1(ky51)
E. coli strain OP50 (saturated culture, previously grown in LB and stored at 4°C)
Pentylenetetrazole (Sigma)
Cell spreader/hockey stick (Thomas Scientific, 7012Q52)
References
Funding
An Undergraduate Creativity and Research Academy grant from The University of Alabama College of Arts & Sciences was awarded to Madeline Vaji for this research.
Reviewed By
Brent NeumannHistory
Received: July 14, 2020Revision received: August 19, 2020
Accepted: August 20, 2020
Published: August 24, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Vaji, MA; Caldwell, GA; Caldwell, KA (2020). Enhanced pentylenetetrazole sensitivity in a C. elegans mutant associated with DNM1 encephalopathy. microPublication Biology. 10.17912/micropub.biology.000296.Download: RIS BibTeX