Biozentrum der LMU München Dept. Biologie I - Botanik Großhadernerstr. 2-4 D-82152 Planegg-Martinsried Germany
Description
Traditional binary shuttle vectors used for creating plant T-DNA insertion mutants can be difficult to work with for several reasons. First, their large size (> 10 kB) and low-copy origin of replication result in low plasmid yields under typical growth conditions. This makes time consuming, and costly, midi or maxi scale plasmid isolation a requirement. Second, accurate sequence information is often not available, rendering vector modification difficult. Finally, cloning techniques that are reliant on restriction enzyme sites prohibit deliberate design of vectors resulting in haphazard configuration of components. Newer cloning techniques, such as Gibson (Gibson et al., 2009) and In-FusionTM assembly, offer unlimited flexibility but rely on sufficient vector fragment quantity; this can be achieved either by restriction digest or by PCR. However, the large size and low DNA yield of binary shuttle vectors make their use in assembly reactions challenging; this is especially true for large batch DNA library cloning.
Movement of features not essential to cloning helped to alleviate the large size of the Ti plasmid; further modification to ‘helper’ plasmids resulted in the pGREEN system (Hellens et al., 1999). The pGREEN binary vector system offers small plasmid size (5.5 kb) and high copy number of the shuttle vector in Escherichia coli (E. coli). Although the pGREEN plasmid collection has aged well, many new molecular tools have been introduced. To provide the research community with an updated toolbox, we created a suite of streamlined pGREEN-based vectors that contain several features frequently used in molecular plant science. We designated the plasmids as pG20, short for pGREEN2020, to maintain a reference to the original hallmark work (Hellens et al., 1999). The small size and high DNA yield provide ease of use, while modular construction allows for easy replacement of the promoter and terminator. The inclusion of a multiple cloning site (MCS) with 6‐bp palindromic unique restriction enzymes sites (Waadt et al., 2008) allows full flexibility with restriction-based cloning, either for ligations or to prepare generic low cost Gibson fragments. To lower design costs further, a single primer pair (or one primer within the pair) can be used to yield several different protein tag fusions. Our vectors contain a variety of C-terminal protein-fusion tags; each one comes with two different antibiotic resistance markers allowing for use in many different plant mutant backgrounds. The addition of N-terminal tags could easily be achieved using the ‘tag less’ construct in a Gibson or In-FusionTM assembly. Plasmids can be obtained from Addgene, the Arabidopsis Biological Resource Center (ABRC), or by contacting our group directly.
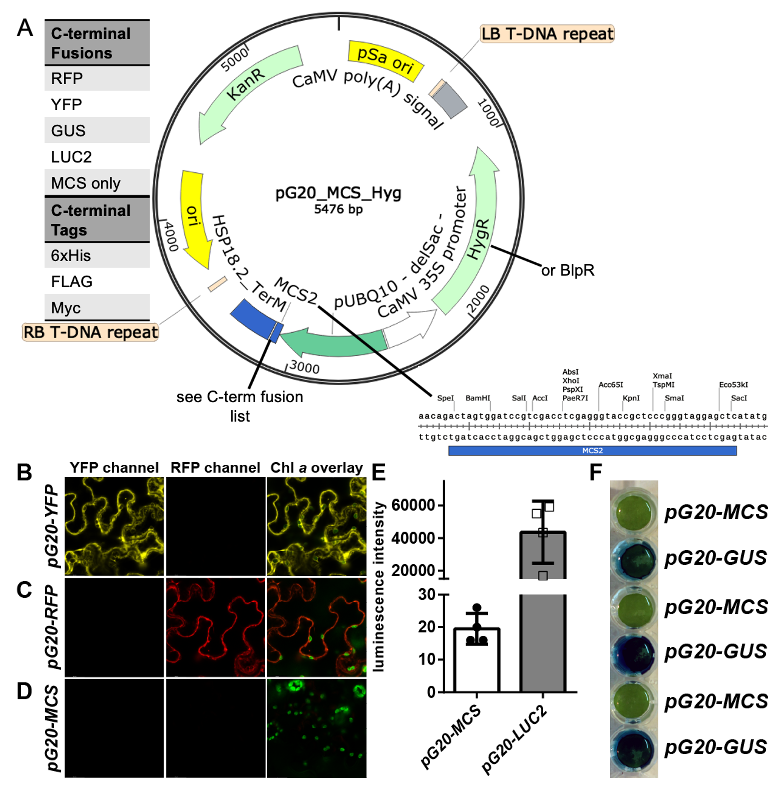
A total of 16 constructs were created, using the constitutive AtUBQ10 promoter in combination with the AtHSP18.2 terminator, resulting in strong, tissue-independent expression. The following C-terminal fusions are available: RFP, YFP, GUS, luciferase2, His-tag, FLAG-tag and Myc-tag. In addition, there is a tag-free construct with only the MCS between promoter and terminator (Figure 1A, image generated using SnapgeneTM software from GSL Biotech). Each plasmid is available with either hygromycin or glufosinate resistance genes, driven by the CaMV35S promoter. Following confirmation of successful cloning by Sanger sequencing, functionality of the newly designed constructs was demonstrated through transient expression in infiltrated Nicotiana benthamiana leaves (Figure 1B-F). Additionally, we have used these constructs to create numerous stable transgenic Arabidopsis thaliana lines in a variety of on-going projects.
Methods
Request a detailed protocolVectors pGreenII 0179 (Hellens et al., 1999) and pHygII-UT-c-term-Venus (Kunz et al., 2014) were used as the initial templates for creation of Gibson assembly fragments via PCR (Gibson et al., 2009). These products were transformed into electrocompetent TOP10 E. coli cells; transformants were selected for on LB media + kanamycin, identified using colony PCR, and verified by Sanger sequencing. Plasmids were isolated using a Qiagen MiniPrep Plasmid kit. The first design yielded pG20_YFP_Hyg, which then served as the parent plasmid for the other hygromycin constructs. Further fragments were either amplified from other vectors: RFP (Waadt et al., 2008), GUS (Nakagawa et al., 2007), LUC2 (Rellán-Álvarez et al., 2015), or synthesized as DNA oligos. The hygromycin constructs were used as templates for the creation of the glufosinate resistance constructs; pFH0032 (Hauser et al., 2013) was used as template to generate the glufosinate resistance gene fragment. The vector suite was then transformed into electrocompetent Agrobacterium tumefaciens GV3101 containing the pSOUP helper plasmid (Hellens et al., 1999). Transformants were selected on LB media + rifampicin, gentamycin, tetracycline, and kanamycin, and confirmed by PCR. All bacterial strains were stored at -80°C in LB + 20% glycerol.
Proof of concept studies were carried out by transient expression via infiltration into four week old Nicotiana benthamiana leaves. Constructs were assayed three days after infiltration. Fluorescence protein localization studies using a Leica SP8 and GUS stains were performed as described before (Höhner et al., 2019). Luciferase activity was measured using a Tecan M200 plate reader using 4 mm leaf punches floated on 100 ml potassium phosphate buffer 100mM (pH 7.0) containing 2 mM D-Luciferin potassium salt (CAS: 115144-35-9).
Reagents
| Primer Name | Sequence | Purpose | Origination |
| HKP590 | tttgttgaaaagtctcaataaagcttcgacgagtcagtaataaacg | Fragment amplification, universal forward – pG20_hyg constructs | This work |
| HKP591 | caaacacatacagcgacttagtttacccgccaatatatcc | Fragment amplification, universal reverse – pG20_hyg constructs | This work |
| HKP592 | tattggcgggtaaactaagtcgctgtatgtgtttgtttgag | Vector amplification, forward – pG20_hyg constructs | This work |
| HKP593 | cgtttattactgactcgtcgaagctttattgagacttttcaacaaagg | Vector amplification, reverse – pG20_hyg constructs | This work |
| HKP867 | gagcagaagctgatctcggaggaagacttgtaagagctcatatgaagatgaagatg | Creation of Myc sequence, forward – pG20_hyg construct | This work |
| HKP868 | ttacaagtcttcctccgagatcagcttctgctccccgggagcggtaccctcg | Creation of Myc sequence, reverse – pG20_hyg construct | This work |
| HKP869 | gactacaaggatgacgatgacaagtaagagctcatatgaagatgaagatg | Creation of FLAG sequence, forward – pG20_hyg construct | This work |
| HKP870 | ttacttgtcatcgtcatccttgtagtccccgggagcggtaccctcg | Creation of FLAG sequence, reverse – pG20_hyg construct | This work |
| HKP871 | catcaccatcaccatcactaagagctcatatgaagatgaagatg | Creation of 6xHis sequence, forward – pG20_hyg construct | This work |
| HKP872 | ttagtgatggtgatggtgatgcccgggagcggtaccctcg | Creation of 6xHis sequence, reverse – pG20_hyg construct | This work |
| HKP881 | ggcatctacttcagatttcggtgacggg | Glufosinate fragment amplification, forward | This work |
| HKP882 | gatcccccctatgagcccagaacgac | Glufosinate fragment amplification, reverse | This work |
| HKP883 | ctgggctcataggggggatcagcttg | Vector amplification, universal forward – pG20_blp constructs | This work |
| HKP884 | cgaaatctgaagtagatgccgaccga | Vector amplification, universal reverse – pG20_blp constructs | This work |
| Construct | NCBI Accession | ABRC stock number | Addgene ID |
| pG20_mCherry_Hyg | MT896402 | CD3-2834 | 159701 |
| pG20_mCherry_Blp | MT896403 | CD3-2835 | 159702 |
| pG20_Venus_Hyg | MT896404 | CD3-2836 | 159703 |
| pG20_Venus_Blp | MT896405 | CD3-2837 | 159704 |
| pG20_GUS_Hyg | MT896406 | CD3-2838 | 159705 |
| pG20_GUS_Blp | MT896407 | CD3-2839 | 159706 |
| pG20_LUC2_Hyg | MT896408 | CD3-2840 | 159707 |
| pG20_LUC2_Blp | MT896409 | CD3-2841 | 159708 |
| pG20_MCS_Hyg | MT896410 | CD3-2842 | 159709 |
| pG20_MCS_Blp | MT896411 | CD3-2843 | 159710 |
| pG20_6xHis_Hyg | MT896412 | CD3-2844 | 159711 |
| pG20_6xHis_Blp | MT896413 | CD3-2845 | 159712 |
| pG20_FLAG_Hyg | MT896414 | CD3-2846 | 159713 |
| pG20_FLAG_Blp | MT896415 | CD3-2847 | 159714 |
| pG20_Myc_Hyg | MT896416 | CD3-2848 | 159715 |
| pG20_Myc_Blp | MT896417 | CD3-2849 | 159716 |
| pGreenII 0179 | EU048866 | ||
| pSoup | EU048870.1 |
Acknowledgments
We thank Drs. David Mendzoa (University of Missouri) and Andrew McCubbin (Washington State University) for providing us with their lab stock of pGREEN 0179. We thank Carsten Voelkner from the Kunz lab for assistance with the Tecan plate reader and GUS images.
References
Funding
This work was funded by a National Science Foundation Career Award (IOS-1553506) to H.-H.K. A.I.P. is grateful for support through the WSU Elling and Higinbotham scholarships and the WSU NASA Space Grant Fellowship.
Reviewed By
Christopher Andrew BrosnanHistory
Received: August 25, 2020Revision received: October 4, 2020
Accepted: October 5, 2020
Published: October 7, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Pratt, AI; Knoblauch, J; Kunz, HH (2020). An updated pGREEN-based vector suite for cost-effective cloning in plant molecular biology. microPublication Biology. 10.17912/micropub.biology.000317.Download: RIS BibTeX