Current address: Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109
Department of Biology, Central Michigan University, Mount Pleasant, MI 48859
Description
In response to adverse environments C. elegans larvae may form stress-resistant and developmentally arrested dauer larvae. Entry into dauer interrupts developmental progression after the second larval molt (Cassada and Russell 1975), and the dauer formation decision must be coordinated with developmental pathways. Heterochronic genes control stage-specific cell fate decisions, and these genes interconnect with the dauer formation (daf) pathway in several ways. Dauer formation and daf genes affect heterochronic phenotypes, and conversely, heterochronic genes can impact dauer by controlling the timing of dauer formation, the morphology of dauer larvae, or the ability to enter dauer (Liu and Ambros 1989; Liu and Ambros 1991; Antebi et al. 1998; Tennessen et al. 2010; Karp and Ambros 2011; Karp and Ambros 2012). Here we find that the lin-41 heterochronic gene regulates the dauer formation decision and dauer morphogenesis via its canonical target, lin-29.
The temperature-sensitive daf-7(e1372) allele results in constituitive dauer entry at 25˚C, and the allele is a useful tool for controlling dauer entry (Vowels and Thomas 1992; Karp 2018). While performing experiments examining the role of lin-41 during dauer, we observed that lin-41(0); daf-7 worms rarely entered dauer. To better understand this phenotype, we quantified the number of dauer and nondauer larvae in synchronous populations of daf-7(e1372) larvae grown at the dauer-inducing temperature of 24˚C.
lin-41(0) mutants are sterile, and therefore the strain must be propagated as a heterozygote (Slack et al. 2000). We took advantage of a closely linked transgene, nIs408[LIN-29::mCherry, ttx-3::gfp] to balance lin-41(0) (Harris and Horvitz 2011). lin-41(0) homozygous larvae were identified by the absence of fluorescence. Controls included nIs408-positive siblings, 2/3 of which should be lin-41(0)/nIs408 and 1/3 of which should be nIs408/nIs408, as well as a strain that is homozygous for the wild-type allele of lin-41.
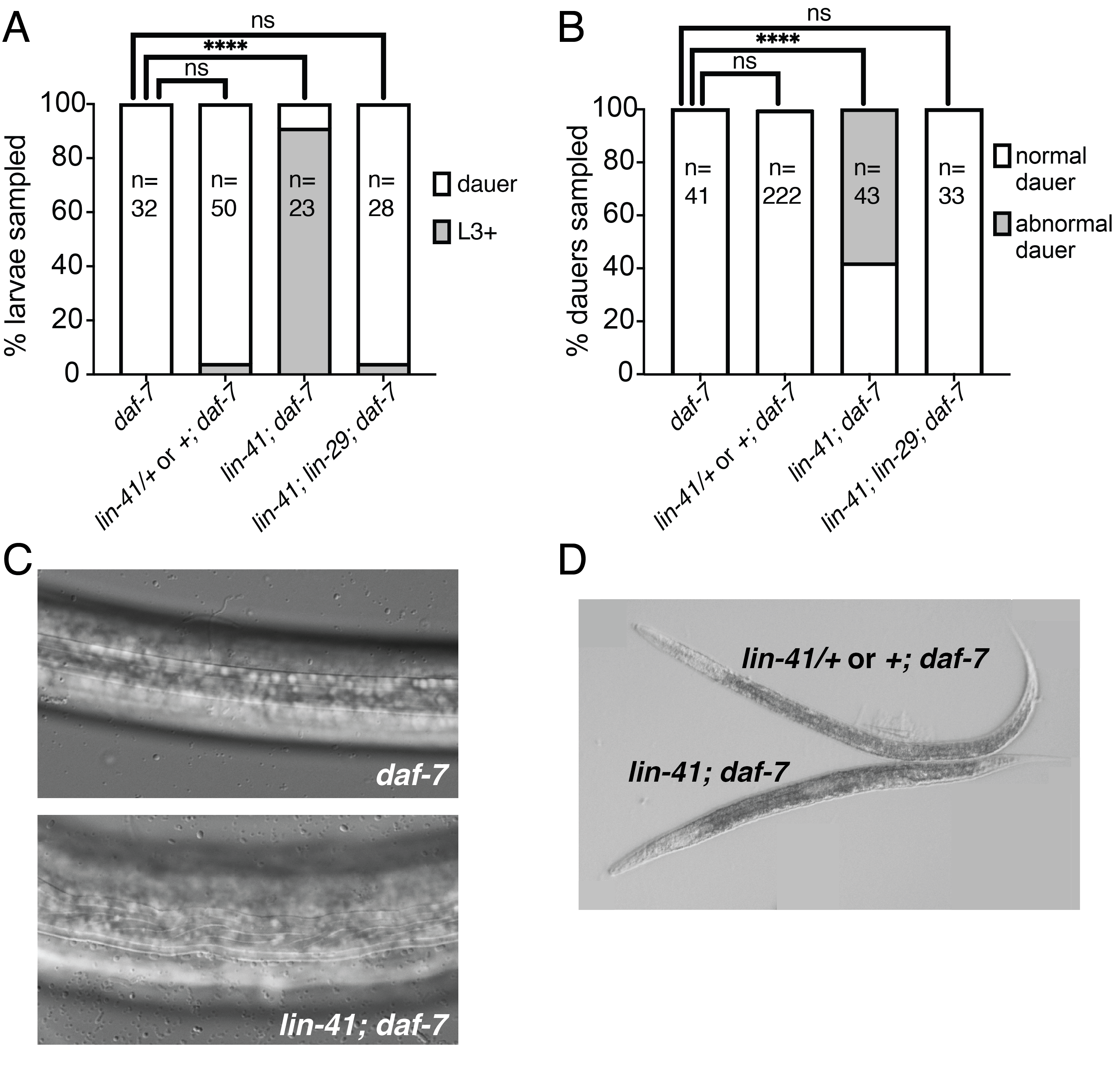
Embryos from each strain were incubated at 24˚C for 50-52 hours to induce dauer formation. As expected, 100% of larvae in the control daf-7 strain were dauers (Fig. 1A). Similarly, the lin-41(0)/nIs408 or nIs408/nIs408; daf-7 larvae formed dauers at high penetrance (96%). By contrast, only 2/23 (9%) of lin-41(0) homozygous larvae were in dauer. The remaining lin-41(0) larvae had continued development and were in the L3 or L4 stage (Fig. 1A). Therefore, lin-41(0) larvae display a dauer formation-defective (Daf-d) phenotype.
The canonical role for lin-41 as a heterochronic gene is to promote larval cell fate in hypodermal cells by directly repressing translation of lin-29 (Slack et al. 2000; Aeschimann et al. 2017). lin-29 encodes a transcription factor that promotes adult cell fate (Rougvie and Ambros 1995). In addition to this canonical role, lin-41 controls several other processes, including oocyte growth and maturation, male tail tip morphogenesis, and a sex-specific neurotransmitter switch in the AIM interneurons, among others. lin-41 acts via lin-29 to control some of these processes, and via other targets to control other processes (Del Rio-Albrechtsen et al. 2006; Spike et al. 2014; Pereira et al. 2019).
To ask whether lin-29 is required for the Daf-d phenotype we observed in lin-41(0) mutants, we examined lin-41(0); lin-29(0) larvae segregating from lin-41(0)/nIs408; lin-29(0) mothers. Because the nIs408 transgene rescues lin-29(0) (Harris and Horvitz 2011), this transgene effectively balances both lin-41 and lin-29. We found that the additional loss of lin-29(0) suppressed the lin-41(0) dauer entry-defective phenotype, as 96% of lin-41(0); lin-29(0); daf-7 larvae examined were in dauer (Fig. 1A).
Since obtaining lin-41(0) dauers using the daf-7 method was inefficient, we asked whether utilization of the natural starvation response could drive lin-41(0); daf-7 larvae into dauer. We allowed each strain to crowd and starve out, and then selected for dauer larvae using 1% SDS (Cassada and Russell 1975). We were able to obtain SDS-resistant lin-41(0) homozygous dauer larvae using this method. Forty-two percent of lin-41(0) dauer larvae displayed normal dauer morphology, as did the two dauer larvae obtained from daf-7 induced dauer formation (Fig. 1A). Interestingly, the remaining 58% of lin-41(0) dauer larvae displayed defective dauer morphology (Fig. 1B). Specifically, both dauer alae and body shape were abnormal. In contrast to the straight ridges that make up wild-type dauer alae, lin-41(0) mutant dauers displayed wavy dauer alae (Fig. 1C). With respect to body shape, there was a range in severity from worms of a normal length but a somewhat lumpy appearance to Dpy dauers that were shorter and fatter than controls (Fig. 1D).
These dauer morphology defects are reminiscent of those caused by loss of either of two other heterochronic genes: lin-14 or lin-28 (Liu and Ambros 1989). However, those defects appear to be due to areas of lateral cuticle that lack dauer alae. Interestingly, the lin-28(0) defect is suppressed by loss of lin-29, whereas the lin-14 defect is not (Liu and Ambros 1989). By contrast, we never observed gaps in dauer alae in lin-41(0) mutants. Furthermore, similarly to the Daf-d phenotype, we found that the dauer morphology phenotype observed in the lin-41(0) homozygotes was suppressed by loss of lin-29, as no lin-41(0); lin-29(0); daf-7 dauer larvae from the SDS experiments displayed defective dauer morphology (Fig. 1B).
In summary, lin-41 regulates two aspects of dauer biology, the decision to enter dauer, and dauer morphogenesis. In both cases, lin-41 appears to act via its canonical target, lin-29. This finding adds to our understanding of the apparent coordination between dauer formation pathways and heterochronic pathways.
Methods
Request a detailed protocolStrains and maintenance.
C. elegans strains were maintained at 20˚C on NGM plates with E. coli strain OP50 as a food source (Brenner 1974).
Dauer-induction via daf-7 and dauer formation assay
Individual gravid adult hermaphrodites from each strain were transferred to 60mm NGM plates and allowed to lay embryos for 3-5 hours at 24˚C. Adults were then removed, and embryos were incubated at 24˚C for an additional 50 to 52 hours. For heterozygous strains, any plates containing homozygous mothers were discarded. Larvae were then imaged as described below. For strains XV238 (lin-41/nIs408; daf-7) and the control VT1777 (daf-7), every larva on the plate was scored for developmental stage. For strain XV239 lin-41/nIs408; lin-29; daf-7), only worms that lacked nIs408 were scored, since nIs408 balances lin-41 and rescues lin-29. Non-dauer larvae were distinguishable from dauer larvae primarily due to the lack of dauer alae, in addition to their larger size, lack of radial constriction, and progression of gonad and vulval development. Six independent trials were performed.
Dauer induction via starvation and dauer morphology assay
Strains were propagated at 20˚C on 60mm NGM plates seeded with OP50. Heterozygous strains were maintained by cloning out individuals and verifying the correct segregation of phenotypes in the progeny. Plates were monitored to identify the point at which the food was exhausted and the point at which dauer larvae appeared. Sets of XV238, XV239, and VT1777 populations that starved at the same time were paired for experiments, to ensure that dauer larvae were approximately the same age between strains within a single experiment. The data in Figure 1B represent 6 independent trials. Dauer larvae were selected using a 30-35 minute incubation in 1% SDS at room temperature. For strain XV239, only larvae lacking fluorescence were analyzed.
Microscopy
Larvae were picked to slides with 2% agarose pads and anesthetized in 0.1 M levamisole. Worms were visualized using a Zeiss AxioImager D2 compound microscope with DIC optics. Representative images acquired using an AxioCam MRm Rev 3 camera and Zen software (2012) from Zeiss are shown. Brightness/contrast was adjusted using Adobe Photoshop.
Reagents
Strains used in this study
| Strain name | Genotype |
| VT1777 | daf-7(e1372); maIs105[col-19::gfp] |
| XV238 | lin-41(n2914)/nIs408[LIN-29::mCherry, ttx-3::gfp]; daf-7(e1372); maIs105 |
| XV239 | lin-41(n2914)/nIs408; lin-29(n546); daf-7(e1372); maIs105 |
Acknowledgments
Thanks to Matthew Wirick (Karp lab) for help with some of the imaging, Anna Zinovyeva (Kansas State University), and Himal Roka (Karp lab) for critical reading of the manuscript, and to WormBase for information. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
References
Funding
NIH R15GM117568 and NSF IOS CAREER 1652283
Reviewed By
AnonymousHistory
Received: October 28, 2020Revision received: November 5, 2020
Accepted: November 9, 2020
Published: November 12, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Cale, AR; Karp, X (2020). lin-41 controls dauer formation and morphology via lin-29 in C. elegans. microPublication Biology. 10.17912/micropub.biology.000323.Download: RIS BibTeX