Institute for Environmental and Gender-Specific Medicine, Juntendo University Graduate School of Medicine, Urayasu, Chiba 279-0021, Japan
Department of Molecular Endocrinology, National Research Institute for Child Health and Development, Setagaya, Tokyo 157-8535, Japan
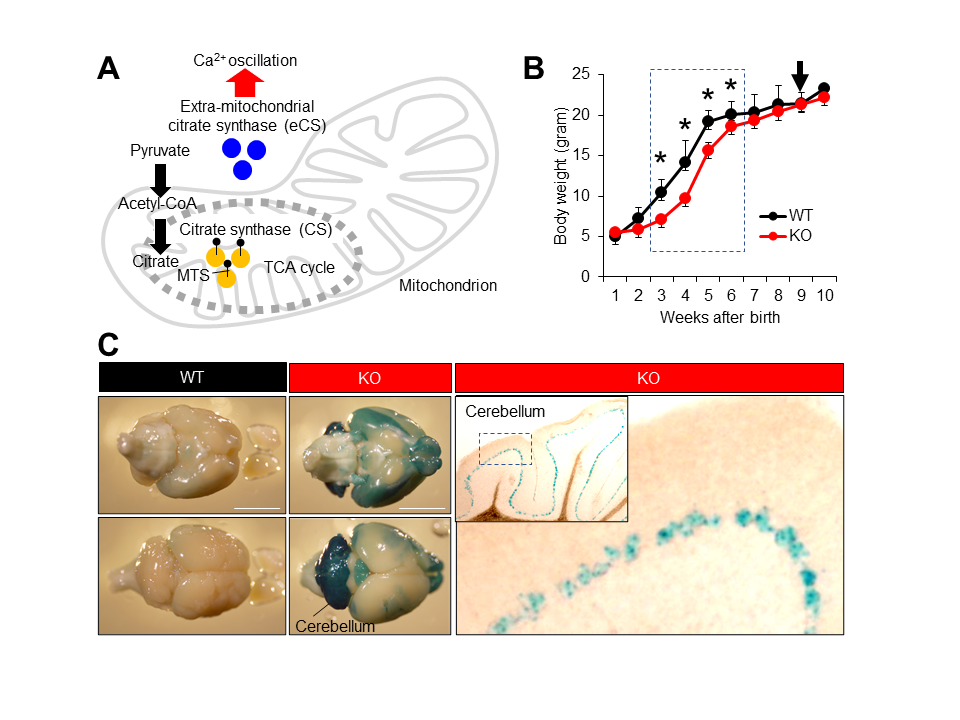
(A) Cooperative function of two citrate synthases, CS and eCS. (B) Growth curve of wild-type (WT) and eCs-KO mice. Ten mice from each genotype were weighed once a week. Dotted box: eCs-KO mice with significantly reduced weight. Arrow: eCs-KO mice brains highlighted with LacZ staining. Values are expressed as mean ± standard error of the mean. * p < 0.0001. (C) The eCs expression in adult mouse brains (9-week-old males). In the eCs-null allele, eCs-promoter drives the expression of the LacZ reporter gene. The LacZ-stained brain was viewed from dorsal (upper panel) and ventral (lower panel) surfaces from the same individual male. In the cerebellum, visualization of β-galactosidase activity in KO adult mice showed the eCs expression in a single cell layer lined inside the cerebellum, presumably the Purkinje cell layer. Dotted box in the inset: enlarged region. Scale bars: 5 mm.
Description
Repetitive increases in the cytoplasmic calcium concentration (Ca2+ oscillation) control a wide variety of biological events (Dupont et al. 2011). In fertilization, a sperm-bearing factor, phospholipase C zeta 1 (PLCz1), triggers Ca2+ oscillation and resumes cell division in eggs. Citrate synthase (CS) is localized to the mitochondrial matrix, where it catalyzes a reaction between acetyl-coenzyme A (CoA) and oxaloacetate to form citric acid (Surpin and Chory 1997) (Figure 1A). The extra-mitochondrial form of CS (eCS) is encoded by a separate gene in mice and is expressed by alternative splicing from the CS gene in humans (Kang et al. 2020). eCS functions as a secondary factor triggering Ca2+ oscillation, which is transferred from the sperm to the eggs (Kang et al. 2020). More specifically, eCS has been found to trigger an initial Ca2+ spike using a PLCz1-independent mechanism. However, the role of eCS-triggered Ca2+ oscillation in broad cell functions is unknown.
Presently, we studied the neuronal involvement of eCS and its neuronal expression using eCs-deficient (KO) male mice carrying the LacZ reporter gene inserted into the eCs-null allele. KO mice were born healthy, but their body size was noticeably small. Therefore, KO mice were weighed daily after birth and compared with wild-type (WT) mice (Figure 1B). From the first to the second week, the body weight was comparable between KO and WT mice. However, KO mice continuously weighed less than WT mice during the third week (6.7 ± 0.5 g vs. 11.2 ± 1.1 g; p < 0.0001), fourth week (9.1 ± 0.4 g vs. 14.0 ± 2.5 g; p < 0.0001), fifth week (15.6 ± 0.6 g vs. 19.5 ± 0.6 g; p < 0.0001), and sixth week (18.3 ± 0.6 g vs. 20.4 ± 0.7 g; p < 0.0001). No difference was noted from the seventh week onward. Moreover, eCS expression was detected in a narrow layer of the cerebellar cortex, probably the Purkinje layer (Figure 1C). From this result, we assumed that eCs and eCS-expressed neuronal cells could regulate the rapid increase in body weight during childhood.
Growth retardation is linked to low concentrations of growth hormone (GH) in humans and mice. GH, also known as somatotropin, is a peptide hormone that mainly functions in growth, cell replication, and cell regeneration (Velloso 2008). GH stimulates the production of the insulin-like growth factor-1 (IGF-1) (also called growth-promoting hormone) to regulate overall body growth (Yakar et al. 2002). Moreover, GH release conclusively depends on changes in intracellular Ca2+ concentration, indicating a critical role of intracellular Ca2+ as a mediator of GH function (Cuttler et al. 1992). Upon the development of the central nervous system, IGF-1 is abundantly expressed in neurons, especially in Purkinje cells, to promote cell survival in the cerebellum (Torres-Aleman et al. 1994; Chrysis et al. 2001), suggesting a possible contribution of eCS to neuronal survival.
Otherwise, spontaneous Ca2+ oscillation is induced in astrocytes both in vitro and in vivo (Zhou et al. 2020). Ca2+ oscillation also controls the fate determination of cultured neural stem cells (Glaser et al. 2020). From our results, we concluded that eCS expressed in the Purkinje cell layer could trigger neuronal signaling via Ca2+ oscillation, subsequently enhancing the rapid growth observed during childhood.
Citrate has been shown to be a regulator of various biological processes, such as insulin secretion (Iacobazzi and Infantino 2014). Citrates act as suppressors of problematic events, such as in the reduction of oxidative stress and inflammation (Iacobazzi and Infantino 2014) and in the protection against traumatic brain injury (Kilbaugh et al. 2015). However, while it is known that citrates are specifically synthesized and released from astrocytes (Westergaard et al. 2017), presently, knowledge of the role of citrates is insufficient. Our results largely contribute to the understanding of eCS-mediated Ca2+ oscillation in the brain.
Methods
Request a detailed protocolAnimals
Mutant mice were generated from C57BL/6-derived embryonic stem cell clones by injection into blastocysts from C57BL/6 mouse with a genetically deleted Csl (eCs) (Csltm1(KOMP)Vlcg; ID14519) obtained from the Knockout Mouse Project (KOMP) repository (an NCRR-NIH-supported strain suppository; www.komp.org). Homozygous mice (C57BL/6 genetic background) were generated by subsequent intercrosses of heterozygous animals. For LacZ staining, 8-week-old male C57BL/6J mice were purchased from Japan SLC Inc. (Shizuoka, Japan) and their brains were used as control.
All mice were housed under specific, controlled pathogen-free conditions. Food and water were available ad libitum. All animal experiments were approved by The Institutional Animal Care and Use Committee of the National Research Institute for Child Health and Development (Experimental number, A2004-004).
LacZ staining
After fixation with 4% paraformaldehyde (Wako Pure Chemical Industries, Osaka, Japan), mouse brains were washed three times with 0.1 M phosphate-buffered saline (pH 7.4) for 5 min. They were then incubated in β-gal staining solution [1 mg/mL 5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside, 2 mM MgCl2, 5 mM potassium hexacyanoferrate (III), and 5 mM potassium hexacyanoferrate (II) trihydrate, 0.01% (w/v) sodium deoxycholate, 0.02 (w/v) NP-40] at 37 °C for overnight. To observe the eCs expression patterns, samples were embedded in Tissue-Tek OCT compound (Sakura, Finetek, Tokyo, Japan), frozen in liquid nitrogen, and cut into thin sections (10 mm) using a cryostat (CryoStar NX70, Thermo Fisher Scientific, Inc., MA).
Statistical analysis
Comparisons were made using one-way analysis of variance following Scheffe’s method, Mann–Whitney U-test, or Fisher’s exact test. Statistical significance was defined as p < 0.05. Results are expressed as the mean ± standard error of the mean.
Acknowledgments
We greatly appreciate the JAC staff for supporting our experiments through comprehensive animal care services. We also acknowledge Editage (https://www.editage.jp/) for English language editing.
References
Funding
This work was supported in part by JSPS KAKENHI, Grant Numbers JP19H01067 and JP19K09793.
Reviewed By
AnonymousHistory
Received: October 8, 2020Revision received: November 10, 2020
Accepted: November 10, 2020
Published: November 12, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Kang, W; Yamatoya, K; Miyado, K; Miyado, M; Miyamoto, Y (2020). Neuronal expression of Ca2+ oscillation initiator is linked to rapid neonatal growth in mice. microPublication Biology. 10.17912/micropub.biology.000325.Download: RIS BibTeX