Description
Cd63 is an adapter protein belonging to the tetraspanin family. As other members, it possesses four membrane-spanning domains, two extracellular loops, and the N- and C-terminus both face the cytoplasm. Cd63 is highly enriched in intraluminal vesicles (ILV) of late endosomes (LE; also called multivesicular bodies, MVB; Termini and Gillette, 2017). Cd63 trafficking to LE occurs via classical routes, i.e. from the ER to the Golgi complex, then to the plasma membrane, from where it is endocytosed and incorporated into ILV (Kobayashi et al., 2000; Pols and Klumperman, 2009). As an ILV-enriched component, Cd63 is a marker of exosomes, as these small extracellular vesicles are created from ILV of certain types of MVB (Pols and Klumperman, 2009; Simons and Raposo, 2009). Finally, Cd63 localizes to early melanosomes, highly specialized organelles that originate from LE-type precursors, and was shown to be required for melanogenesis in human melanoma cells (Basrur et al., 2003; van Niel et al., 2011). In our preceding analysis (Kreis et al., 2020), using Xenopus embryos we found the orthologous gene expressed in the trunk neural crest (TNC) and in melanosome-associated tissues such as the retinal pigment epithelium and melanophores (pigment producing cells of poikilotherm vertebrates; Raposo and Marks, 2007). Neural knockdown of cd63 specifically caused eye morphogenesis defects in early tadpole stages, surprisingly without impacting TNC formation and migration, nor melanophore specification and melanogenesis (Kreis et al., 2020).
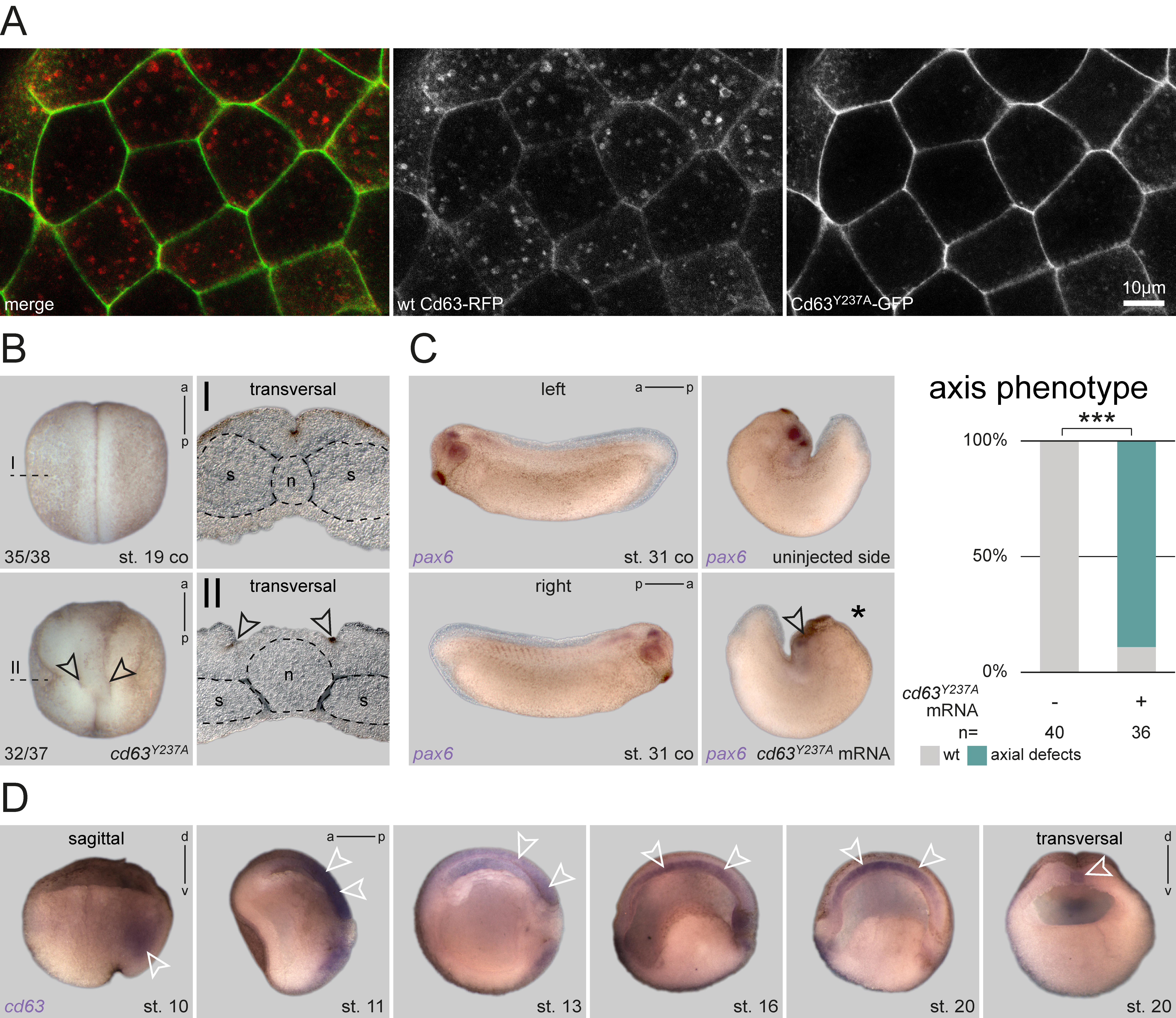
To analyze the cellular basis of this eye phenotype, we asked whether endosomal localization of Cd63 was required for correct eye development. Endocytosis and incorporation of Cd63 into ILV is mediated by a C-terminal ‘lysosomal targeting motif’, comprised of the last five amino acids (AA), GYEVM (Rous et al., 2002). Single AA mutagenesis combined with indirect immunofluorescence screening had determined a tyrosine-to-alanine replacement (GYEVM to GAEVM) to be most efficient in preventing translocation from the plasma membrane to LE in rat kidney cells (Rous et al., 2002). We generated two equivalent constructs using Xenopus cd63: one comprising the mutated coding sequence (cd63Y237A/CS2+), and a second one fused N-terminally to eGFP (cd63Y237A/eGFP/CS2+) for localization testing. To verify that the Xenopus mutant construct mimicked the published mis-localization in embryos in vivo, we injected mRNA of cd63Y237A/eGFP together with an RFP-tagged wt cd63 (Lee et al., 2007) into the animal hemisphere and compared their subcellular localization by confocal microscopy. While WT Cd63 localized to both plasma membrane and endosomal structures as published (Lee et al., 2007), mutant Cd63Y237A was not found in endosomal structures, but was strongly enriched in the plasma membrane (Figure 1A). Thus, our construct provided a valuable tool to analyze the function of Cd63 localization to LE during Xenopus development.
Next, we injected the construct into the dorsal lineage of 4-cell stage embryos to analyze potential phenotypes. Surprisingly, injected embryos developed strong axial phenotypes during neurulation, resulting in an open neural tube at late neurula stages. Transversal sections of such specimens at the trunk level revealed that bilateral hinge points elevating the neural folds had formed correctly. However, the floorplate between these hinge points was extremely wide, preventing neural fold apposition and fusion (Figure 1B). Additionally, the notochord appeared amorphic in these specimens, not displaying a regular round shape as in control embryos, i.e. notochord morphogenesis was severely compromised. Together, the observed phenotype indicated a failure of proper axial elongation. This phenotype became even more pronounced, when injected embryos were reared to tailbud stages. Even in unilaterally injected specimens, axial extension of dorsal tissues was significantly inhibited, resulting in an extreme dorsal curvature in most embryos (Figure 1C). When such embryos were processed for in situ hybridization (ISH) to highlight eye development using a pax6 probe, optic pax6 expression was strongly reduced on the injected side (Figure 1C), indicating impairment of eye morphogenesis, and reminiscent of the morpholino (MO)-mediated knockdown of cd63 (Kreis et al., 2020).
As these phenotypes suggested a possible role of Cd63 in tissues important for axial convergent extension (CE) behavior (Keller and Sutherland, 2020), we next performed ISH to analyze the expression patterns of cd63 during gastrulation and neurulation. cd63 was expressed in both the axial mesoderm and the neuroectoderm (Figure 1D), i.e. germ layers exhibiting CE and extensive axial elongation. In bisected embryos, neural plate expression was visible during late gastrulation and early neurulation and vanished afterwards. Mesodermal expression was found continuously throughout this embryonic time-frame. cd63 was first expressed in the deep mesoderm during early gastrulation, later, during formation and elongation of the notochord, these tissues showed intense mRNA enrichment as well. Together, these results support the conclusion that localization of Cd63 to LE is involved in regulation of embryonic axial extension during late gastrulation and/or early neurulation.
While the observed phenotype is striking, it also reveals a significant difference to our previous cd63 loss-of-function approach (Kreis et al., 2020). By inhibiting translation, we found cd63 to be required for eye morphogenesis, with aberrant optic vesicle formation during late neurulation being the first visible phenotype. While such knockdown embryos did not show early axis elongation phenotypes, both approaches resulted in later eye defects. Therefore, the early defects presented here, indicate a dominant-inhibitory effect caused by plasma membrane retention of Cd63 (cf. Figure 1A). Therefore, the lack of endosomal trafficking of Cd63Y237A, which partially mimics the loss of cd63, could indicate a specific requirement for correct eye morphogenesis. In this work, we have not directly assessed if Cd63Y237A mislocalized to the plasma membrane in the dorsal mesoderm or posterior neural ectoderm as well, i.e. in those tissues performing axis elongation behavior. However, if this was the case, as expected, this might also result in aberrant accumulation of Cd63 binding partners, which in turn caused Cd63Y237A-specific interference with CE behavior in these early stages.
Tetraspanins are well-known to serve as multipurpose adapters by forming ‘tetraspanin-enriched microdomains’. In this way, they have been reported to influence cell adhesion properties, and thus to alter cellular morphology and migratory behavior (Termini and Gillette, 2017). Supporting this notion, Cd63 is considered a negative driver of advanced stages of melanoma, as it inhibits epithelial-mesenchymal transition (EMT), and thus invasive migratory behavior (Radford et al., 1997; Jang and Lee, 2003; Lupia et al., 2014). The role of Cd63 in cell migration is also associated with its well-known regulatory function on integrin abundance at the membrane (Pols and Klumperman, 2009). In turn, correct integrin function and localization has been demonstrated to be required for CE movements in Xenopus (Marsden and DeSimone, 2003; Davidson et al., 2006; Keller et al., 2008). Thus, Cd63Y237A might indirectly inhibit axial elongation and CE by negatively influencing integrin dynamics, potentially also in tissues normally not expressing cd63 endogenously (i.e. in the neural plate during late neurula stages). In this context, it is important to mention that the observed phenotype (Figure 1B) is highly reminiscent of a CE defect as observed after blocking dishevelled (dvl) function specifically in the neural ectoderm. Upon dvl perturbation, neural folds also show apical constriction and medial movement at mid neurulation, but CE is not occurring in the midline in between (Wallingford and Harland, 2002). Dvl regulates CE in both floorplate and notochord by participating in the planar cell polarity pathway (PCP) via its PDZ protein interaction domain (Wallingford and Harland, 2001). Interestingly, Cd63 possesses a PDZ-binding motif in its C-terminus, which has been demonstrated to bind the PDZ domain of Syntenin-1 (Latysheva et al., 2006; Pols and Klumperman, 2009). Syntenin-1, on the other hand, has been shown to interact with PDZ-binding domains of Syndecans, and Syndecan-4, a well-known regulator of PCP, is also involved in recycling of integrins, i.e. sharing a similar role with Cd63 (Grootjans et al., 1997; Muñoz et al., 2006; Morgan et al., 2013). Future analyses will reveal whether there is a direct or indirect interaction of Cd63 with Dvl or Syntenin-1, which would also explain its effect on axial elongation during Xenopus neurulation.
Methods
Request a detailed protocolXenopus laevis care and maintenance
Frogs were purchased from Nasco (Fort Atkinson, WI, USA). Handling, care and experimental manipulations of animals was approved by the Regional Government Stuttgart, Germany (V349/18ZO ‘Xenopus Embryonen in der Forschung’), according to German regulations and laws (§6, article 1, sentence 2, nr. 4 of the animal protection act). To induce ovulation, female frogs were injected subcutaneously with 300-700 units of human chorionic gonadotropin (Sigma). Embryos were staged according to Nieuwkoop and Faber (1994). Only clutches of healthy embryos from healthy females were used for the experiments shown. Embryos from one batch were randomly picked and used as control or tested specimens.
Construct cloning, mRNA synthesis and microinjections
To generate an endosome-excluded version of cd63, the full-length X. laevis L-homeolog was isolated and the lysosomal targeting motif GYEVM was mutated to GAEVM by changing the codon TAT to GCT using the following primer pairs:
F1_5’-GAATTCCATGGCGGTGGAAGG-3’ (for both plasmids)
R1_5’-CTCGAGCGGTCACATGACTTCAGCTCCA-3’ (for CS2+ plasmid)
R2_5’-CTCGAGCGGTCCCATGACTTCAGCTCCA-3’ (for CS2+/c-terminal eGFP plasmid)
PCR products were introduced into CS2+ or CS2+/c-terminal eGFP plasmids using EcoRI and XhoI enzymes. For mRNA synthesis, plasmids were linearized with NotI and transcribed in vitro (Sp6 polymerase) using Ambion message machine kit. Drop size was calibrated to 4 nl per injection. 560pg (for double injections in B) or 400pg (for single injections in A, C) of mRNA was injected.
In situ Hybridization
ISH probes for X. laevis cd63 was used as described (Kreis et al., 2020). For in situ mRNA detection, ISH was performed after fixation in MEMFA for 2-3 h at room temperature and processed following a standard protocol (Sive et al., 2000). RNA in situ probes were transcribed using SP6 or T7 polymerases.
Embryo sections
For vibratome sections (thickness: 30-35 μm), embryos were embedded in a glutaraldehyde-crosslinked gelatin-albumin mix (embedding medium: 2.2 g gelatine, 135 g bovine serum albumin, 90 g sucrose dissolved in 450 ml PBS) and razor blade-sectioned as indicated in whole-mount panels using a Leica VT1000S vibratome.
Photo Documentation
Confocal images of fluorescence were taken with a Zeiss LSM 700 and adjusted using the Zeiss Zen 2012 Blue edition. Embryo pictures were taken with a Zeiss SteREO Discovery.V12 (whole embryos), or an Axioplan2 (sections) microscope using AxioVision 4.6. Adobe Photoshop CS6 was used for cropping and careful brightness adjustments. All figures were arranged using Adobe Illustrator CS6.
Statistical analysis
Statistical calculations were performed using Pearson’s chi-square test. *=p<0.05, **=p<0.01, ***=p<0.001 were used.
Acknowledgments
We like to thank V. Andre, A. Schäfer-Kosulja and S. Vogel for technical assistance, and the Blum Lab members for valuable feedback. The pax6 plasmid was a kind gift of Dr. T. Hollemann, the rfp-cd63/CS2 plasmid of Dr. N. Kinoshita.
References
Funding
J.K. was a recipient of a Ph.D. fellowship from the Landesgraduiertenförderung Baden-Württemberg.
Reviewed By
AnonymousHistory
Received: August 26, 2020Revision received: November 17, 2020
Accepted: September 22, 2020
Published: November 27, 2020
Copyright
© 2020 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Kreis, J; Bonß, R; Feistel, K; Vick, P (2020). Expression of an endosome-excluded Cd63 prevents axis elongation in Xenopus. microPublication Biology. 10.17912/micropub.biology.000334.Download: RIS BibTeX