Abstract
Vertebrate embryonic development is regulated by a few families of extracellular signaling molecules. Xenopus laevis embryos offer an excellent system to study the cell-cell communication signals that govern embryonic patterning. In the frog embryos, Wnt/β-catenin plays a pivotal role in regulating embryonic axis development, and modulation of the Wnt pathway is required for proper antero-posterior patterning. Recently, a novel secreted, organizer-specific Wnt inhibitor, Bighead, was identified that acts by downregulating Lrp6 plasma membrane levels. Here, I describe a method to purify biologically active Bighead protein and confirm that Bighead promotes Xenopus head development.
Description
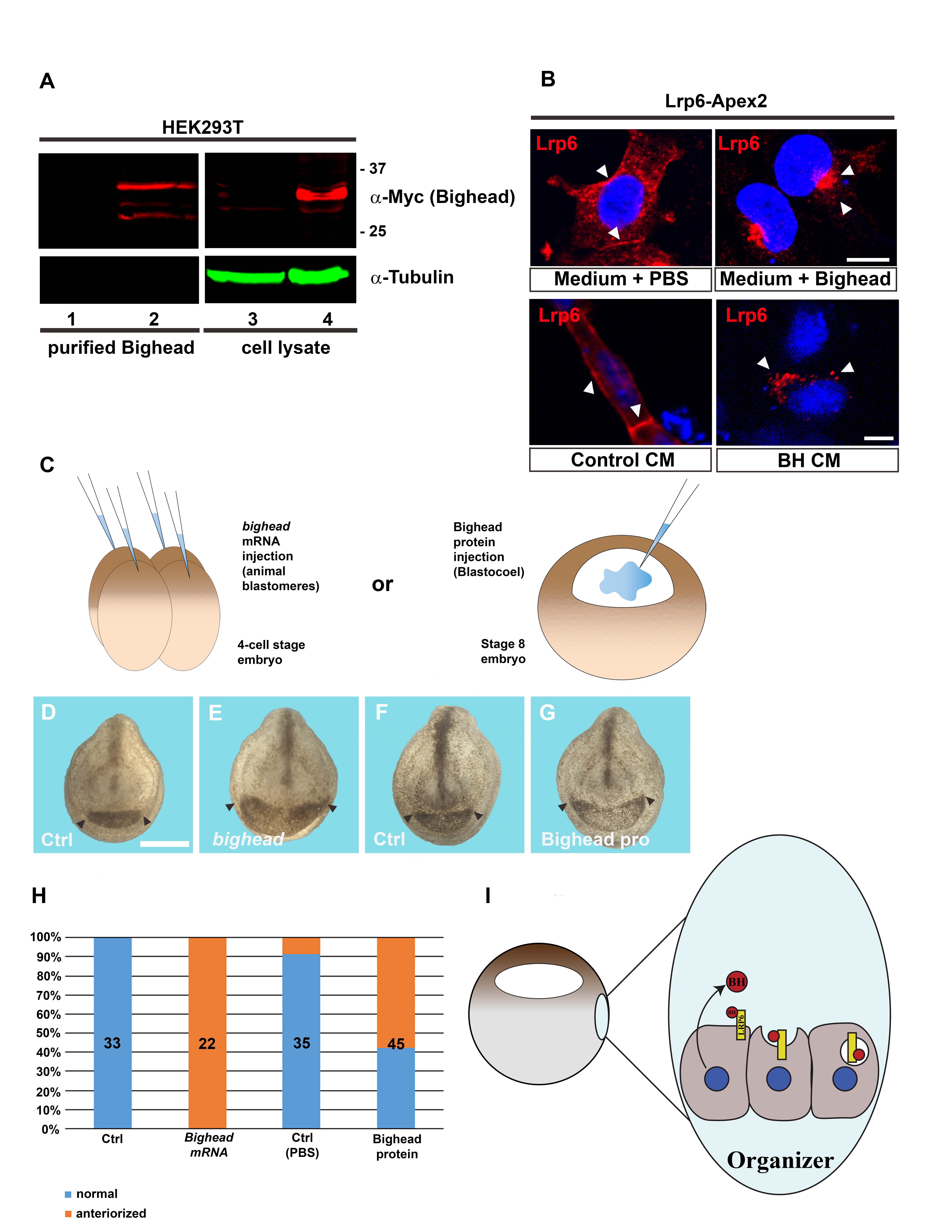
Previous RNA-sequencing experiments were performed on Xenopus laevis embryos to reveal novel Spemann organizer-specific genes. Analysis on the dorsal and ventral transcriptomes of early frog gastrula revealed an “organizer signature” of genes dorsally expressed and under the control of maternal Wnt signaling (Ding et al., 2017a and b). Among the new genes found in the dorsal side of Xenopus, we identified an additional member of the Dapper antagonist of β-catenin (Dact) family of proteins, called dact4 (Colozza and De Robertis, 2020) as well as several secreted Wnt antagonists, including vlk (Vertebrate lonesome kinase, also known as Pkdcc) (Ding et al., 2017a) angiopoietin-like 4 (Kirsch et al., 2017) and bighead (Ding et al., 2018). The latter represents the only member of a new family of secreted factors conserved in frogs and fishes, but absent in higher vertebrates such as mammals (Ding et al., 2018). Interestingly, protein structure prediction indicated relevant homology with the prodomain region of the latent form of myostatin/growth and differentiation factor 8 (GDF8) (Ding et al., 2018). Xenopus bighead (gene ID 108715768) encodes a Wnt antagonist that binds to the Lrp6 co-receptor and induces its internalization and degradation into lysosomes, downregulating canonical Wnt/β-catenin signaling. Overexpression of bighead mRNA into Xenopus embryos promotes the enlargement of head, eyes and cement gland, accompanied by expanded anterior neural markers, such as rx2a, foxg1 and otx2 (Ding et al., 2018). On the other hand, morpholino-mediated knock-down of bighead has the opposite effect, as shown by the strong reduction in head development and expression of markers such as otx2. Altogether, these evidences suggest that bighead is required for head development in Xenopus embryos via inhibition of Wnt signaling (Ding et al., 2018). Here, I show that secreted Bighead protein purified from the extracellular medium maintains its activity, as assessed in cell culture and Xenopus embryos. To this aim, I used a Streptag/Streptactin-based purification system, which harness the ability of a short tag (8 amino acid long) to bind strongly to modified streptavidin molecules (Maier et al., 1998; Schmidt et al., 1996; Voss and Skerra, 1997). Streptag was introduced at the C-term end of Bighead, where modifications are well tolerated and do not interfere with its signaling activity (Ding et al., 2018). The Streptag was also preceded by a Myc tag, to allow for easier detection of Bighead protein. pCS2 plasmid containing recombinant bighead-Myc-Streptag DNA was transfected into HEK-293T cells. Conditioned medium (CM) containing Bighead protein was collected 48 hours after transfection, and applied to Streptactin columns for affinity purification. Media from empty pCS2 transfected cells was used as a control. Eluted protein was then dialyzed and concentrated in PBS using Millipore concentrators. Western blot analysis confirmed expression and purification of Bighead protein (Fig. 1A). Notably, two major bands were observed between 25 and 37 kDa, suggesting possible post-translational modifications. 200 ng of protein were then applied to the culture medium of HEK-293T cells expressing Lrp6-Flag-APEX2 (Colozza et al., 2020). Compared to control, Lrp6-Flag-APEX2 relocated in intracellular clusters resembling endosomal vesicles, within 3 hours after treatment with purified Bighead protein (Fig. 1B). Interestingly, both Bighead CM and purified protein showed similar effect on Lrp6 subcellular localization, supporting the specificity of Bighead activity. Then, I turned to Xenopus embryos to further characterize the activity of Bighead-Streptag (Fig. 1C). mRNA injections induced strong enlargement of the head and cement gland (Fig. 1D, E), a phenotype that is associated with inhibition of zygotic Wnt signaling (Niehrs, 2001; Ding et al., 2018). Interestingly, injection of the purified protein into the blastocoel (a cavity that collects extracellular fluids and growth factors and plays important role in embryonic patterning) of stage 8 blastula embryos produced similar effects (Fig. 1F-H). This result confirms that purified Bighead protein is active by operating at the extracellular level, as expected for a secreted factor, and promotes head enlargement in Xenopus laevis. This also suggests a possible mechanism for Bighead function in frog embryos, where extracellular Bighead protein (produced and secreted by cells in the Spemann organizer) attenuate Wnt signaling by inducing Lrp6 endocytosis, as proposed in the model in Fig. 1I.
In conclusion, this study provides a straightforward and user-friendly method to purify extracellular soluble factors that can be applied directly to cultured cells and vertebrate embryos to study cell-cell signaling modulation.
Methods
Request a detailed protocolCloning and mRNA transcription. Xenopus laevis bighead cDNA was subcloned into a Gateway-adapted pCS2 contaning Strep-Tag (for C-terminal tagging). pCS2-Bighead Step-Tag was digested with NotI restriction enzyme. 1 μg of the linearized plasmid was used as a template for mRNA in vitro transcription, using the Sp6 mMessage mMachine and following manufacturer’s instructions.
Xenopus husbandry and embryo injection. All animal experiments were performed in accordance to guidelines for animal welfare. X. laevis frogs were purchased from the Nasco Company. A sperm suspension was obtained from testicles manually dissected from male frogs, and crushed in 1 ml of 1x Marc’s Modified Ringers (MMR, 0.1 M NaCl, 2.0 mM KCl, 1 mM MgSO4, 2 mM CaCl 2,5 mM HEPES, pH7.4). Ovulation of female frogs was induced the night before experimentation by injecting 800 Units of human chorionic gonadotropin (hCG). The day after, frogs were let to spontaneously lay eggs in a high-salt solution (1.2 x MMR). Laid eggs were collected and fertilized with 200–300 μl of the sperm suspension. To remove the jelly-coat, fertilized eggs were treated with a 2% cysteine in 0.1x MMR solution pH 7.8, for about 7 min at room temperature (RT). Dejellied embryos were then cultured in 0.1x Marc’s modified Ringer’s solution and staged according to Nieuwkoop and Faber (1967). For mRNA injection, 200 ng of Bighead Strep-Tag mRNA were injected animally in each blastomere of 4-cell stage embryos. For protein injection, Xenopus embryos at blastula stage were injected into the blastocoele cavity with 40 nl Bighead Strep-Tag protein solution (250 ng/ml, in PBS). Embryos were then collected at stage 22-24 and analyzed for phenotype.
Bighead protein expression and purification. pCS2 Bighead Step-Tag was transfected into HEK 293T cells using Lipofectamine 2000 and following manufacturer’s instructions. 16 hours after transfection culture medium was replaced with fresh one, and cells were cultured for further 96 hours. The conditioned media from 5 transfected 10 cm-plates was collected and run through Strep-Tactin columns. Columns were washed and Bighead Strep-Tag protein was eluted following manufacturer’s instructions. Eluted protein was concentrated using Amicon concentrators (Merck Millipore), exchanging the eluent with PBS. Bighead protein was quantified using a BCA assay kit and a BSA (bovine serum albumin) standard curve. Medium from empty vector transfected cells was used as a negative control, and processed as described above.
Reagents
– Lipofectamine 2000 (Thermo Fisher Sci., 11668030)
– Strep-Tag/Strep-Tactin purification kit (IBA Lifesciences)
– Flag antibody, mouse monoclonal (Sigma, M2 F1804); RRID:AB_262044
– anti-Myc antibody, rabbit polyclonal (GeneTex, GTX103436); RRID:AB_11162914
– anti alpha-Tubulin antibody, mouse monoclonal (Santa Cruz Biotech, sc-32293); RRID:AB_628412
– secondary antibodies for immunofluorescence: Cy3 donkey anti-mouse (Jackson ImmunoResearch, 715-165-150); RRID:AB_2340813
– secondary antibodies for infrared Western Blot: IRDye 680LT donkey anti-rabbit IgG (Li-Cor, 926-68023; RRID:AB_10706167); IRDye 800CW donkey anti-mouse 800 (Li-Cor, 926-32212; RRID:AB_621847)
– precast gels for SDS-PAGE, Novex WedgeWell 4-20%, Tris-Glycine, 1.0 mm, Mini Protein Gel, 10-well (Thermo Fisher Sci., XP04200BOX)
– mMessage mMachine, Sp6 in vitro transcription kit (Thermo Fisher Sci., AM1340)
Acknowledgments
I thank Eric Sosa for helping with the drawing shown in Figure 1 I.
References
Funding
Gabriele Colozza is the recipient of a Lise Meitner postdoctoral fellowship
Reviewed By
Douglas HoustonHistory
Received: November 28, 2020Revision received: December 30, 2020
Accepted: January 4, 2021
Published: January 5, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Colozza, G (2021). Purified Bighead protein efficiently promotes head development in the South African clawed frog, Xenopus laevis. microPublication Biology. 10.17912/micropub.biology.000347.Download: RIS BibTeX