Abstract
RNA interference is a widely conserved mechanism of gene regulation and silencing across eukaryotes. In C. elegans, RNA silencing is coordinated through perinuclear nuage containing at least four granules: P granules, Z granules, Mutator foci, and SIMR foci. Embryonic localization of these granules is known for all except SIMR foci. Here we establish that SIMR foci first appear at the nuclear periphery in the P4 germline blastomere and become numerous and bright in the Z2 and Z3 progenitor germ cells. This timing coincides with the appearance or de-mixing of other germline granules, providing further evidence for coordinated germ granule reorganization.
Description
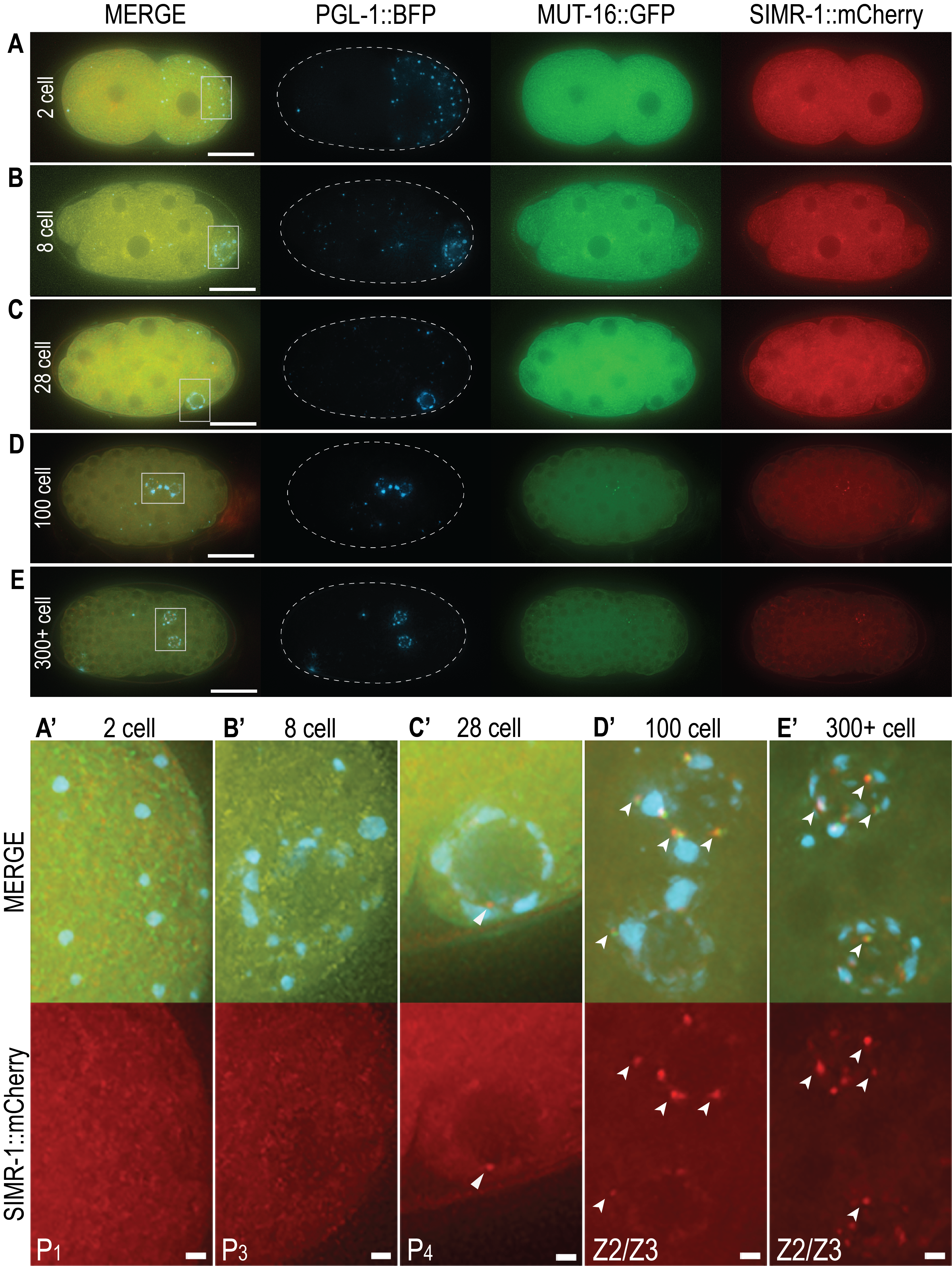
Multiple condensates occupy the perinuclear space of C. elegans germ cells, where they coordinate RNA surveillance to ensure proper gene expression (Lev and Rechavi 2020; Sundby et al. 2021). The most well-studied of these condensates are P granules, phase-separated germ granules required for maintenance of germ cell identity and fertility (Kawasaki et al. 1998; Updike et al. 2014). P granule morphology and localization is well documented in C. elegans development (Strome et al. 1982). Adjacent to P granules are Mutator foci, which are nucleated by MUT-16 and required for the amplification of small interfering RNAs (siRNAs) to create a robust and heritable silencing signal (Phillips et al. 2012). During development, faint Mutator foci are occasionally seen in the P4 germline blastomere of 30-cell embryos, but are most robust and numerous in the Z2 and Z3 progenitor germ cells (PGCs) of 100-cell embryos (Uebel et al. 2020). A third germline condensate, Z granules, are situated between P granules and Mutator foci and facilitate transgenerational epigenetic inheritance of silencing signals. Z granule components ZNFX-1 and WAGO-4 colocalize with P granules in early embryos, but begin to de-mix from P granules in the Z2/Z3 PGCs to form separate Z granule condensates (Wan et al. 2018). Lastly, recently discovered SIMR foci also intimately localize within this cluster of germline granules. SIMR-1, a key component of SIMR foci, is a Tudor domain protein that mediates production of secondary siRNAs for piwi-interacting RNA (piRNA)-targeted mRNAs (Manage et al. 2020). While P granule, Z granule, and Mutator foci localization through embryonic development has been previously described, the embryonic appearance of SIMR foci is not known.
Here we use endogenously tagged SIMR-1::mCherry to investigate the embryonic onset of SIMR foci. We further visualize embryonic P granules with PGL-1::BFP and Mutator foci with MUT-16::GFP to compare fluorescence and interaction with SIMR foci. Live imaging of embryos reveals diffuse cytoplasmic expression of SIMR-1 in all stages (Figure 1A-E), similar to previous observations of MUT-16 expression (Uebel et al.. 2020). Because SIMR foci are present in the germlines of adult hermaphrodites and localize adjacent to P granules, we focused our analysis on the germline blastomeres and progenitor germ cells of embryos (Figure 1A’-E’). In the 2-cell embryo, P granules segregate to the posterior P1 germline blastomere, yet no punctate SIMR foci are present (Figure 1A, A’). Similarly, no SIMR foci are found in 8-cell embryos as P granules begin associating with nuclear pores in the P3 germline blastomere (Figure 1B, B’). The 28-cell embryo yields the first observable SIMR focus adjacent to perinuclear P granules in the P4 germline blastomere (Figure 1C, C’). While we consistently observe SIMR foci in 28- to 50-cell embryos (n = 3), these foci are few and faint. Around the 100-cell stage, the P4 cell gives rise to the Z2 and Z3 PGCs, and it is here that we reliably observe bright and numerous SIMR foci (Figure1D, D’). These bright foci also persist in the Z2/Z3 of late-stage embryos of 300 or more cells (Figure1E, E’). Our data reveals the previously unknown embryonic appearance of SIMR foci.
Consistent with their localization in adult germ cells, embryonic SIMR foci appear adjacent to both P granules and Mutator foci. Interestingly, the appearance of fewer, faint SIMR foci in the P4 cell and more numerous, bright SIMR foci in the Z2/Z3 PGCs is similar to the timing of Mutator foci formation in embryos (Uebel et al.. 2020). Both the de-mixing of Z granules and the appearance of robust Mutator foci and SIMR foci in the PGCs correlates with the onset of embryonic germline transcription (Seydoux and Dunn 1997, Wan et al. 2018, Uebel et al. 2020). Taken together, this observation suggests that the arrival of newly produced mRNAs in the Z2/Z3 PCGs may necessitate or facilitate the coordinated reorganization of germ granule components for efficient RNA surveillance.
Methods
Request a detailed protocolMicroscopy
Worms were grown at 20°C according to standard conditions (Brenner 1974). Gravid adult C. elegans were dissected in 10 µL M9 to expose embryos and mounted on a fresh 2% agarose pad for live imaging. At least 3 embryos were observed for each stage. All images were acquired with a DeltaVision Elite (GE Healthcare) microscope using a 60x N.A. 1.42 oil-immersion objective. Ten 0.2-micron Z stacks were compiled as maximum intensity projections and pseudo-colored using Adobe Photoshop to create each image. The same exposure, acquisition, and pseudo-coloring settings were used for each image.
Reagents
USC1401 simr-1(cmp15[simr-1::mCherry::2xHA]) mut-16(cmp3[mut-16::gfp::3xFLAG]) I; pgl-1(cmp226[pgl-1::bfp::3xFLAG]) IV.
Strain Construction
USC1401 was created by crossing USC1269 (pgl-1(cmp226[pgl-1::bfp::3xFLAG])) (Uebel and Phillips 2019) and USC774 (simr-1(cmp15[simr-1::mCherry::2xHA]) mut-16(cmp3[mut-16::gfp::3xFLAG]) I; unc-119(ed3) III) (outcrossed) (Manage et al. 2020). All strains are available upon request.
Acknowledgments
We would like to thank members of the Phillips Lab for helpful discussions of the manuscript.
References
Funding
This work was supported by the National Institute of Health grants R35 GM119656 (to C.M.P.), the National Science Foundation Graduate Research Fellowship Program Grant No. DGE 1418060 (to C.J.U.) and the University of Southern California Research Enhancement Fellowship (to C.J.U.). C.M.P. is a Pew Scholar in the Biomedical Sciences supported by the Pew Charitable Trusts (www.pewtrusts.org) and C.J.U. is a USC Dornsife-funded Chemistry-Biology Interface trainee. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reviewed By
Dustin UpdikeHistory
Received: February 16, 2021Accepted: February 22, 2021
Published: February 22, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Uebel, CJ; Manage, KI; Phillips, CM (2021). SIMR foci are found in the progenitor germ cells of C. elegans embryos. microPublication Biology. 10.17912/micropub.biology.000374.Download: RIS BibTeX