Iowa Neuroscience Institute, University of Iowa
Abstract
Oxidative stress is thought to be a major contributor to aging processes. Here, we report differential effects on neurotransmission caused by loss-of-function mutations of Superoxide dismutase 1 (Sod1) and by paraquat (PQ) feeding in Drosophila. We demonstrated alterations in Sod1 mutants; the larval neuromuscular junction displayed supernumerary discharges and the adult giant-fiber escape pathway showed increased latency and poor response to repetitive high-frequency stimulation. Even though the concentrations used led to motor coordination defects and lethality, PQ feeding failed to reproduce such performance deficits in these larval and adult preparations, indicating mechanistic distinctions between these genetic and pharmacological manipulations of oxidative stress.
Description
Free radicals, such as the superoxide anion (O2–) impart oxidative stress upon an animal and are thought to be a major contributing factor to age-related changes in the nervous system (Finkel & Holbrook, 2000; Harman, 1956; Harman, 1981). In eukaryotes, the cytosolic enzyme Cu2+/Zn2+ Superoxide Dismutase (in Drosophila encoded by Sod1, originally cSOD, Campbell et al., 1986) is an important free radical scavenger that converts superoxide into hydrogen peroxide. Drosophila Sod1 loss-of-function mutants exhibit elevated levels of reactive oxygen species (ROS) combined with a drastically shortened adult lifespan (median ~11 d. vs 50 d. for wild-type flies at 25 °C, Phillips et al., 1989) and reduced locomotor ability in larvae (Şahin et al., 2017) and adults (Ruan & Wu, 2008). Coupled with these longevity and behavioral phenotypes, several neuromuscular deficits have recently been identified in Sod1 loss-of-function mutants including deranged nerve and synapse morphology in larvae (Milton et al., 2011) and adults (Agudelo et al., 2020; Şahin et al., 2017), as well as disrupted neurotransmission along the giant-fiber (GF) jump-and-flight escape circuit (Iyengar et al., 2020; Iyengar, 2016; Ruan, 2008). Given the striking phenotypes, a question that arises is: To what extent the straightforward assumption holds that aspects of the Sod1 phenotypes can be recapitulated by pharmacologically induced oxidative stress?
Here, we compare neuromuscular physiology in Sod1 loss-of-function (‘null’) mutants to wild-type (WT) flies with elevated oxidative stress levels induced by paraquat (PQ) feeding. A widely used herbicide, PQ is a well-studied drug often utilized experimentally to generate superoxide anions and induce oxidative stress in Drosophila (Parkes et al., 1993; Shukla et al., 2014). We examined synaptic transmission at the abdominal neuromuscular junction (NMJ) of 3rd instar Sod1 larvae to WT larvae fed on PQ-containing medium from egg hatching. In adults, we compared the performance of the GF escape pathway (Engel & Wu, 1992) in Sod1 mutants to PQ-fed WT flies. The PQ feeding concentrations chosen for this study (5 or 10 mM throughout the larval stage; and 10 mM in adults for 4 d) were reported to induce lethality and behavioral abnormalities in WT flies within a 3 – 7 d window (Arking et al., 1991; Jahromi et al., 2013). Over this period, we indeed observed a marked loss of motor coordination. Flies often “stumbled” while walking and occasionally exhibited wing-buzzes upon gentle “tapping” of the vial. The PQ concentrations selected for exposing larvae throughout their development was near lethality levels, beyond which few larvae survived. Nevertheless, our neurophysiological findings reveal that PQ-fed WT individuals clearly do not replicate all Sod1 phenotypes in both larval and adult physiological recordings.
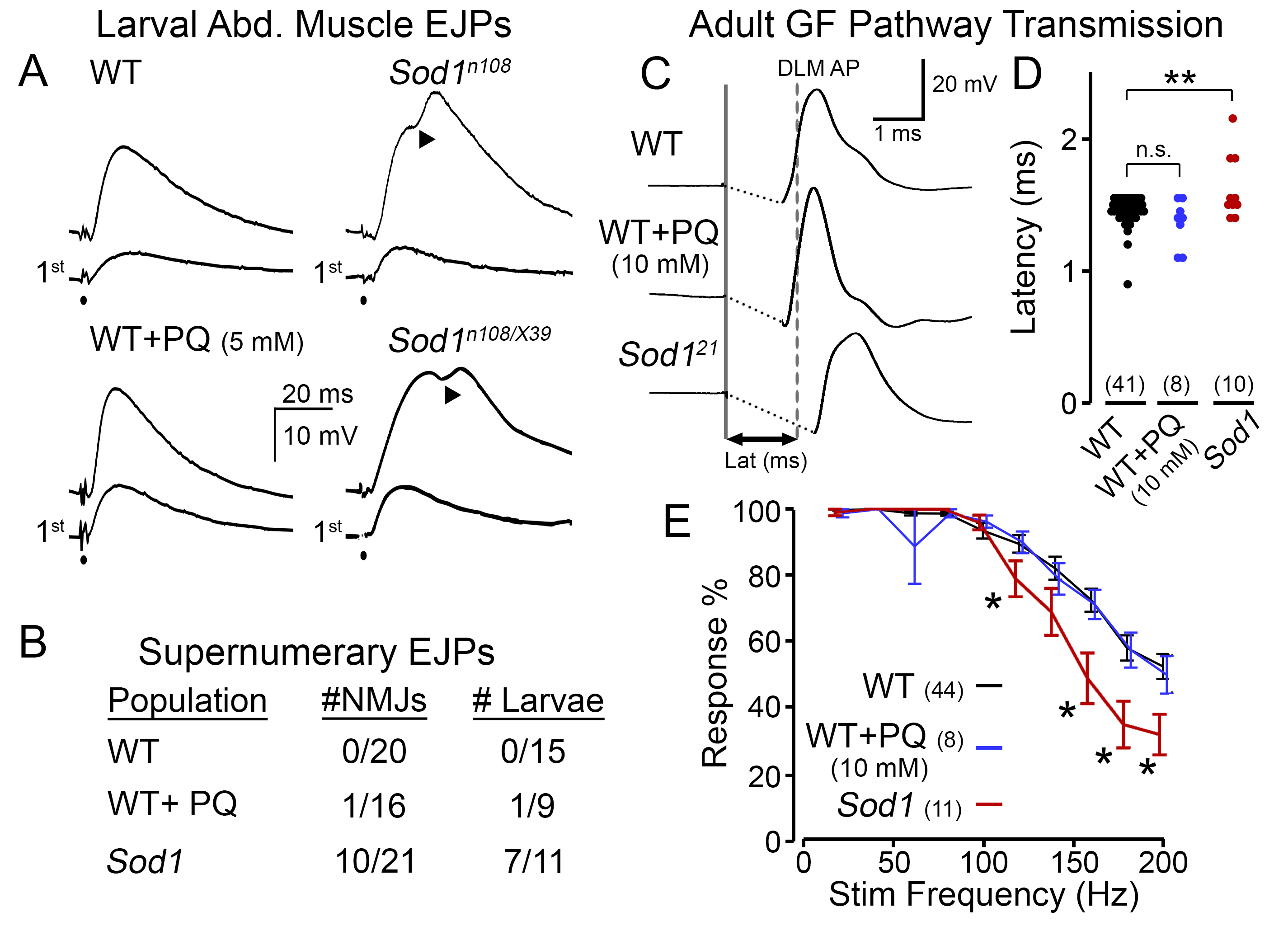
At the larval NMJ, we found Sod1 mutant larvae displayed a striking phenotype which was absent in WT counterparts (Figure 1A). Although an initial nerve stimulation produced similar excitatory junctional potentials (EJPs, analogous to EPSPs) in WT and Sod1 larvae (lower traces), after repetitive stimulation (at ~15 Hz, upper traces), we observed extra ‘humps’ in the Sod1 waveforms indicative of supernumerary EJPs (representative EJPs from a homozygous Sod1n108 and hetero-allelic Sod1n108/Sod1X39 individuals are shown). These abnormal events indicate the stimuli recruit secondary action potentials in the motor nerve (Ueda & Wu, 2009) and suggest a form of nerve hyperexcitability in Sod1 mutants. In contrast, PQ-fed WT flies displayed EJPs that were remarkably similar to control diet-fed counterparts in terms of shape and amplitude. Indeed, across the population of larvae, extra ‘humps’ in the EJP waveform were commonly observed in Sod1 larvae but were exceedingly rare in PQ-fed WT or control-fed WT flies (Figure 1B).
To compare the effects of PQ feeding and Sod1 mutations on adult nervous system function, we examined stimulus-response relationships of the GF pathway. In this circuit, established visual and mechanosensory inputs trigger spiking in the GF neuron in response to visual looming or air pressure stimuli (Card & Dickinson, 2008; Lehnert et al., 2013; Mu et al., 2014; Trimarchi & Schneiderman, 1995). The descending GF axons project directly to the tergotrochanteral (TTM, ‘jump muscle’) motor unit and indirectly via the peripherally synapsing interneuron (PSI) to the dorsal longitudinal muscle (DLM, ‘flight muscle’) motor unit (Gorczyca & Hall, 1984; King & Wyman, 1980). Direct electrical stimulation of the GF neuron triggers an action potential in the DLM with a stereotypic latency in WT flies (~1.4 ms, Figure 1C), known as ‘short-latency’ responses (Engel & Wu, 1996). Consistent with previous observations (Iyengar et al., 2020), we found Sod1 flies displayed an overall increase in latency with a wider variability compared to WT counterparts (Figure 1C-D). However, PQ-fed WT flies did not show such retarded responses; instead latencies displayed were remarkably similar to control WT individuals. We also examined the ability of the GF pathway to follow trains of high-frequency stimulation (20 – 200 Hz) in PQ-fed flies and Sod1 mutants (Figure 1E). At stimulation frequencies higher than 100 Hz, we found Sod1 displayed a progressively worse ability to follow stimulation compared to WT counterparts (largely consistent with observations using a twin-pulse refractory protocol, c.f. Iyengar et al., 2020). However, across all frequencies, PQ-fed WT flies displayed a similar response rate compared to control diet-fed individuals.
Our observations at the larval NMJ and along the GF pathway in adults reveal clear and consistent defects in neurotransmission among Sod1 loss-of-function mutants. It should be noted that mutant flies harboring an amyotrophic lateral sclerosis (ALS)-associated allele (G85R) has been reported to display a collection of neuromuscular defects, including NMJ overgrowth and moderately increased spontaneous miniature EJPs, which can be further compared with PQ-feeding effects in future studies (Held et al., 2019). The Sod1 alleles we studied, i.e. Sod1n108, Sod1X39 and Sod121, were independently isolated (Phillips et al., 1989; Şahin et al., 2017). Thus, it is most likely Sod1 disruption, rather than contributions from secondary ‘background’ mutations, gives rise to the phenotypes. We observed poor motor coordination and lethality, indicating our PQ-feeding indeed produced effects consistent with previous reports (Arking et al., 1991). Notably, even though the larval NMJ and adult GF pathway are remarkably robust systems, studies from our group and others have demonstrated clear electrophysiological alterations in Sod1 loss-of-function (this study; see also: Watson, et al., 2008, Iyengar, et al., 2020). In contrast, pharmacological manipulation of oxidative stress levels with standard PQ-feeding protocol failed to recapitulate Sod1 mutant phenotypes, suggesting the neuromuscular deficits in Sod1 flies described here do not arise by a simple overall elevation of superoxide levels. One possible contributing factor is altered regulation of superoxide anions in specific cell types or subcellular compartments leading to the complexity or diversity of Sod1 mutant phenotypes. Alternatively, mutations in Sod1 or PQ-exposure can induce different patterns of downstream adjustments involving altered activity of other redox enzymes (Missirlis et al., 2003) and of different 2nd messenger signaling pathways such as BMP signaling (Berke et al., 2013; Held et al., 2019), Nrf2/KEAP1 and/or NF-KB, (Sies et al., 2017; Wang et al., 2018).
Methods
Request a detailed protocolFly Stocks and Drug Feeding
The Sod1 (originally cSOD, Campbell et al., 1986; also known as Sod, Ruan & Wu, 2008) hypomorphic/null mutant alleles examined included: Sod1n108 (also known as Sod1n1) [FBal0015933]; Sod1X39 [FBal0030258] (Campbell et al., 1986; Phillips et al., 1989); and Sod121 [FBal0343866] (Şahin et al., 2017). We studied homozygous n108, and 21 individuals; however, we examined the hetero-allelic n108/X39 combination due to the poor viability of homozygous X39 flies (Ruan & Wu, 2008). Phenotypes among the Sod1 alleles were remarkably similar. Therefore, the data was pooled for statistical analyses (Figure 1B, D & E). The WT strain was Canton-Special. For adult experiments, we studied WT and Sod1 populations before significant age-dependent mortality (< 1% and 5% mortality, 7-8 d for WT and 2-3 d Sod1). For detailed characterization of the age-dependence of GF performance in Sod1 and WT flies, see Iyengar et al. (2020).
Flies were reared on Frankel & Brosseau (1968) cornmeal-based media (see Kasuya et al., 2019) at room temperature (23 °C). To make PQ-laced media, measured amounts of paraquat (Sigma #856177) and Blue #1 dye (to indicate mixing, flavorsandcolor.com, Diamond Bar, CA) were added to melted fly media to achieve final concentrations of 5 or 10 mM PQ and 25 μg/ml dye. For larval feeding experiments, larvae were reared on PQ media (5 mM or 10 mM) from egg hatching until the post-feeding 3rd instar stage. Adults were aged to 3 – 4 d. and were then fed with PQ-containing (10 mM) medium for 4 d and used for recording.
Larval Neuromuscular Electrophysiology
Neuromuscular recordings from post-feeding 3rd instar larvae are described in (Ueda & Wu, 2006, 2009). Dissections were performed in Ca2+-free HL3 saline (Stewart et al., 1994). Excitatory junction potentials (EJPs) were recorded from abdominal muscles in HL3.1 saline (Feng et al., 2004) containing relatively low Ca2+ levels (0.1 or 0.2 mM). To evoke nerve action potentials and EJPs, the segmental nerve was severed form the ventral ganglion and stimulated through the cut-end with a suction electrode (~10-μm inner diameter). The stimulation pulse train (0.1 ms stimuli delivered at 15 Hz for 50 s) was generated from a Grass S88 stimulator (West Warwick, RI) through a custom-made isolation unit. The stimulation voltage was adjusted to be double the EJP threshold value to ensure motor axon stimulation. To record EJPs, a glass intracellular microelectrode filled with 3 M KCl (~ 60 MΩ resistance) was inserted in the abdominal muscle # 6/7 (Crossley, 1979). Signals were recorded by a DC amplifier (model M701, WPI, Sarasota, FL and an additional custom-built amplifier) and digitized using a data acquisition card (USB 6212, National Instruments, Austin TX) and pClamp5 (Molecular Devices, Burlingame, California, USA) controlled by a PC.
Adult Giant-Fiber Physiology
Examination of the giant-fiber pathway physiology were performed in tethered flies (see Engel and Wu (1992); Iyengar and Wu (2014) for details). Flies were anesthetized on ice, affixed to a tungsten pin with cyanoacrylate glue, and given ~30 min to recover. To record spiking from the DLM, an electrolytically sharpened tungsten electrode was inserted into the attachment site of the dorsal-most fiber (#45, see Miller, 1950). A similarly constructed electrode was inserted into the abdomen as reference. Electrical activity was picked-up by an AC amplifier (gain: 100x, bandwidth: 10 – 10,000 Hz, AM systems Model 1800) and digitized by data acquisition card (USB 6212, National Instruments) controlled by a PC running LabVIEW (2018, National Instruments).
Electrical stimulation to activate the GF pathway was delivered by an isolated pulse stimulator (AM systems Model 2100) via sharpened tungsten electrodes inserted in each cornea. Stimuli consisted of brief pulses (0.1 ms duration, 30 V amplitude) which reliably activated the GF neuron and downstream elements (‘short-latency responses’ in Engel & Wu, 1996; Iyengar et al., 2020). The GF latency (Figure 1D) was determined as the interval between stimulation and the DLM spike reaching its half-maximum height. The frequency following ability of the GF pathway was determined by delivering trains of 10 pulses at increasing frequencies (20 Hz – 200 Hz in 20 Hz increments) with a 5-s interval between trains. For each pulse train, the number of DLM responses divided by number of stimuli was reported.
Statistical Analysis
All statistical analysis was done in MATLAB (r2020b, MathWorks). Kruskal-Wallis non-parametric ANOVA tests (with Holm-Bonferroni corrected rank-sum post hoc analysis) were employed to establish statistical significance in Figure 1D and E. * p < 0.05, and ** p < 0.01.
Acknowledgments
We thank the undergraduate research assistants in the Wu Lab for their help in maintaining fly stocks and assisting with experiments. This work was supported by an NIH Grants (AG 051513 and NS 111122 to CFW). AI was supported by an Iowa Neuroscience Institute Fellowship.
References
Funding
NIH - AG051513, NS111122 to CFW; Iowa Neuroscience Institute fellowship to AI
Reviewed By
AnonymousHistory
Received: March 5, 2021Revision received: March 29, 2021
Accepted: March 31, 2021
Published: April 13, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Ueda, A; Iyengar, A; Wu, CF (2021). Differential effects on neuromuscular physiology between Sod1 loss-of-function mutation and paraquat-induced oxidative stress in Drosophila. microPublication Biology. 10.17912/micropub.biology.000385.Download: RIS BibTeX