Abstract
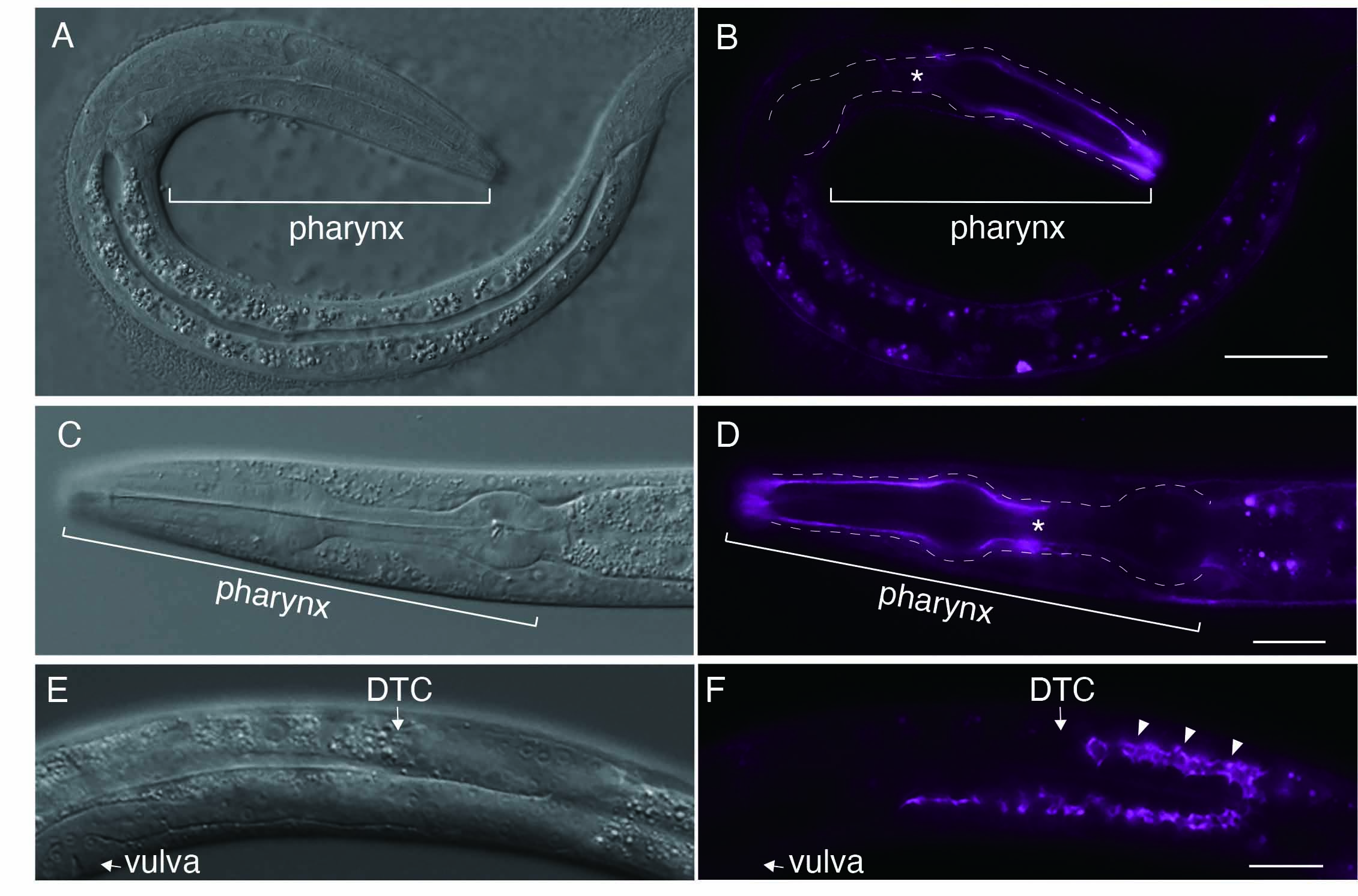
The gene him-4 encodes the C. elegans homolog of hemicentin, an evolutionarily conserved extracellular matrix protein. Despite being an extracellular matrix protein, mutations in him-4 result in pleiotropic defects during development and demonstrate tissue fragility. While previous studies in C. elegans have confirmed the localization of either transgenic HIM-4::GFP or endogenous C-terminally tagged HIM-4::mNeonGreen, only green or green/yellow fluorescent protein-tagged HIM-4 are available to the C. elegans community. Here, I used CRISPR/Cas9 technology to insert the far-red fluorescence protein mKate2 at the him-4 genomic locus, and established worms expressing mKate2::HIM-4. As expected, localization of mkate2::HIM-4 at the rachis was observed at the L4 stage. In contrast to the localization of type IV collagen or nidogen such as major components of the basement membrane, mkate2::HIM-4 was polarized at the anterior part of the pharyngeal basement membrane. A unique polarized localization pattern of pharyngeal basement membrane is maintained throughout the L1–L4 stages.
Description
The gene him-4 in Caenorhabditis elegans encodes hemicentin, which is evolutionally conserved (Vogel & Hedgecock, 2001). Hemicentins are characterized by multiple domains, including a conserved von Willebrand A domain, a long chain of immunoglobulin modules, a series of EGF-like modules, and a carboxyl-terminal fibulin-type module(Whittaker & Hynes, 2002). Despite being an extracellular matrix protein, mutant alleles of hemicentin exhibit both pleiotropic defects during development and tissue fragility (Hodgkin et al., 1979; Vogel & Hedgecock, 2001). HMCN1, a human ortholog of him-4, is involved in age-related macular degeneration 1(Thompson et al., 2007). Previous studies in C. elegans have shown that HIM-4 is secreted from the skeletal muscle and gonads and is recruited to specific extracellular sites using a transgenic strain (Vogel & Hedgecock, 2001). A recent study reported the construction of a C-terminally endogenous mNeonGreen-tagged him-4 using CRISPR/Cas9 genome editing (Keeley et al., 2020). Using the bright far-red fluorescence protein mKate2, I generated a homozygous mkate2::HIM-4 strain, where mKate2 is inserted after the 109th glycine residue of the him-4 genomic locus via CRISPR/Cas9 genome editing. As expected, localization of mkate2::HIM-4 to the pharyngeal basement membrane was observed at the L1 stage (Figure 1B). In contrast to the uniform localization on the basement membrane of type IV collagen or nidogen, which are major components of the basement membrane, that of mkate2::HIM-4 was polarized at the anterior part of the pharyngeal basement membrane (Kang & Kramer, 2013; Matsuo et al., 2019)(Figure 1B). A unique polarized localization pattern was also observed in the pharyngeal basement membrane at stage L4 (Figure 1D). These observations indicate that polarized HIM-4 localization in the anterior part of the pharyngeal BM is maintained throughout the L1–L4 stages, although it remains unclear whether polarized localization is associated with pharynx function. The localization was also observed at the rachis during gonadal development (Figure 1F) at the L4 stage; this observation was consistent with that of previous reports (Vogel & Hedgecock, 2001). As the repellent behavior of C. elegans is observed after exposure to blue light (488 nm) that excites GFP but not after exposure to light (588 nm) that excites red fluorescent protein(Ward et al., 2008), live imaging may be useful for visualizing mkate2::HIM-4 localization in vivo.
Methods
Request a detailed protocolAnimals were cultured on standard NGM plates with E. coli (OP50) at 20 °C. The N2 (Bristol) strain was used as the injection strain. To generate a genome-edited mKate2 knock-in him-4 locus, the pDD287 vector was used as the repair template. pDD287 was modified to generate the N-terminal mkate2::self-exiting cassette system repair template using the following primers: 5ʹ him-4 fwd AACGACGGCCAGTCGGATGACAAAAATGACCCTACT, 5ʹ arm him-4 rev GGCTCCCGATGCTCCTCCGTGCACGTACACTTTACTG, 3ʹ arm him-4 fwd AGCGAGGAAGACTTGGGAGGTGATTGTCCAGAGAAG, and 3ʹ arm him-4 rev CTATGACCATGTTATCATAAATGAATGAGGACGGTAA. Underlines highlight homology arms of him-4 gene. The DNA sequences of all the constructed plasmids were confirmed via Sanger sequencing. The pDD162 (Peft-3::Cas9 + Empty sgRNA) vector (Addgene plasmid # 47549) was used for Cas9 expression (Dickinson et al., 2013). The sgRNA plasmid was derived from Addgene Plasmid 46169. For direct cleavage of the target sequence, the following sgRNA sequence was used for him-4: 5ʹ AAAGTGTACGTGCACGGA/GG 3ʹ. To prevent re-digestion after knock-in of the him-4 locus, a fluorescence mKate2 tag was inserted immediately before the position of the PAM sequence for the sgRNA sequence (/) at him-4. The sgRNA vectors were microinjected together with 50 ng/µL pDD162, 50 ng/µL sur-5::gfp, and 50 ng/µL repair template in N2 animals. Single mkate2::HIM-4 was isolated based on the roller phenotype and sur-5::GFP expression, and the SEC was excised as described previously (Dickinson & Goldstein, 2016). All images were acquired using an Axiocam 506 mono, mounted on a Zeiss AxioImage M2 microscope, equipped with 20× or 40× Plan Apochromat objective lenses, and controlled using ZEN 2.3 pro (Zeiss). All images were optimized and superimposed using Photoshop 2021 (Adobe Systems).
Strain: IHR-187 xyz18[mkate2::HIM-4]X
It will be made available at the CGC.
References
Funding
This work was supported by research grants from Ohsumi Frontier Science Foundation and JSPS KAKENHI Grant Numbers JP19K06552
Reviewed By
David MatusHistory
Received: March 26, 2021Revision received: April 9, 2021
Accepted: April 12, 2021
Published: April 29, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Ihara, S (2021). N-terminally endogenous mKate2-tagged HIM-4 in Caenorhabditis elegans. microPublication Biology. 10.17912/micropub.biology.000386.Download: RIS BibTeX