Abstract
The utility of single copy transgenic insertions in C. elegans is often limited by low expression. We examined the effects of modifying the trans-splicing signal, the Kozak ribosome binding site, the N-terminal amino acid of the reporter and the 3′ UTR sequences on the expression level of a mec-4 promoter GFP transgene. The trans-splicing signal and the 3′ UTR had most dramatic effects on expression while modifying the Kozak signal or the N-terminal amino acid had less influence on expression.
Description
Several methods are currently available to create single copy C. elegans transgenic animals that express recombinant proteins under the control of specific promoters. Unfortunately, in many cases these transgenic animals express the engineered tools at levels too low to be practically used in routine experiments. We examined the effects of modifications to the trans-splicing sequence, the Kozak ribosomal binding site that promotes translational initiation (Kozak 1987), the N-terminal amino acid controlling N-end rule-mediated protein stability, and the 3′ UTR sequences which regulate message stability on steady state GFP levels in a set of single copy insertions at the same position. Our results document that modifying each of the three components can influence expression levels.
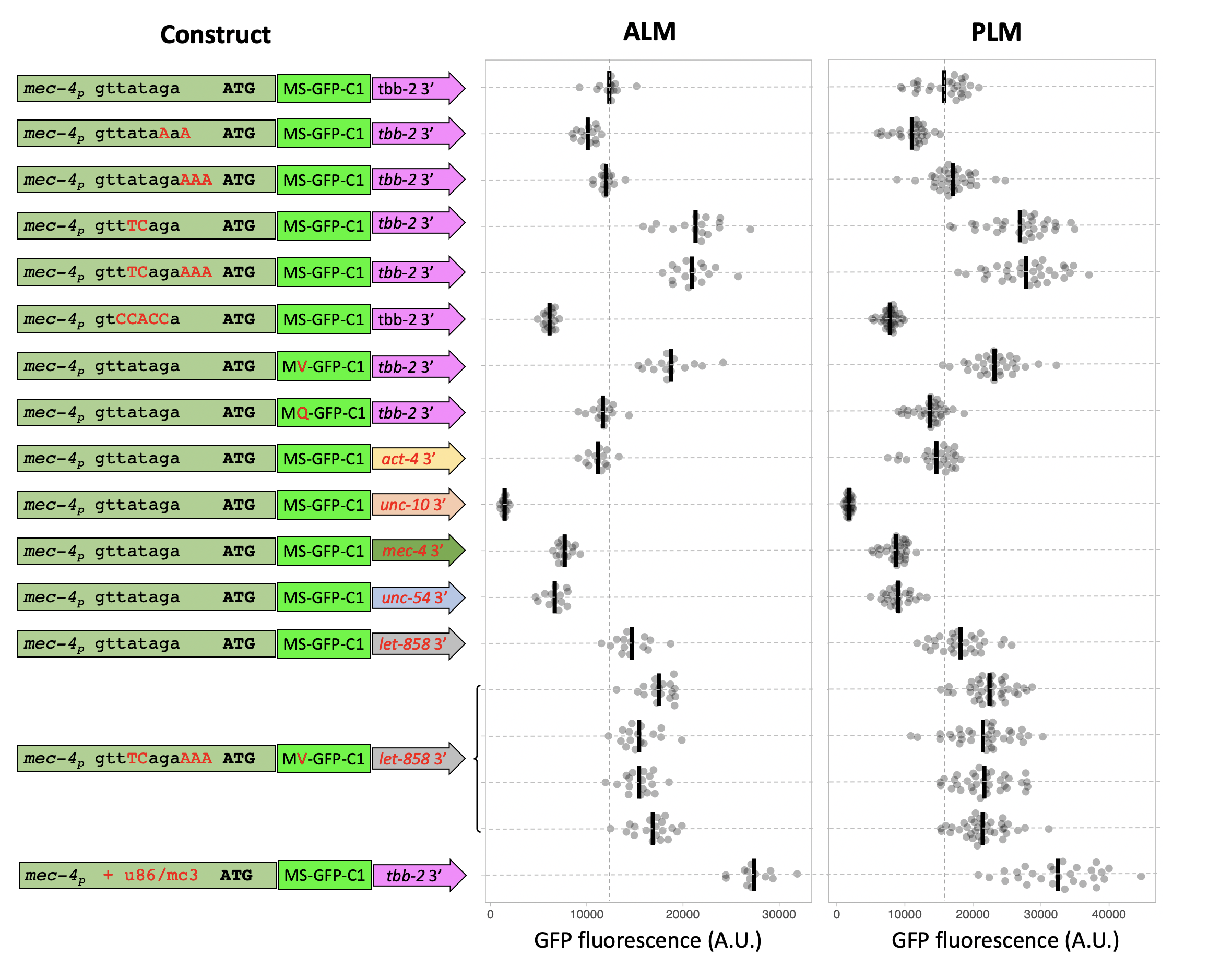
We used an efficient RMCE protocol (Nonet 2020) to create the transgenic animals. Modified versions of a mec-4 promoter GFP-C1 tbb-2 3′ UTR DNA construct were created in an RMCE integration vector using a Golden Gate cloning approach, then integrated using a standard injection protocol. After outcrossing the expression level of GFP at steady state in L4 animal PLM and ALM soma was quantified (Figure 1).
Modification of the sequence upstream of the ATG to contain a computationally determined optimal C. elegans consensus Kozak sequence (Blumenthal and Steward 1997) reduced the steady state level of GFP. However, since the replacement also alters the trans-splice acceptor, we also inserted 3 A bases to add a consensus Kozak site without disrupting the splicing signal. This modification had no influence on expression. Modification of the trans-slice acceptor sequence from TTATAG to the consensus TTTCAG increased steady state levels ~ two fold, consistent with studies that have shown disruption of the trans-splice signal reduces translation efficiency in vivo (Yang et al. 2017). Disrupting the trans-splice signal by mutating to a non-consensus sequence in the -1 to -5 sites (TCCACC) had an opposing effect reducing expression level about two-fold.
Protein expression levels are also regulated by the N-terminal sequence of proteins, through a biological process known as the N-end rule (Gonda et al. 1989). Modification of the first post-Met amino acid of the GFP-C1 protein coding sequence improved expression when the amino acid was changed to valine, the most stabilizing amino acid, and reduced slightly when changed to the unfavorable amino acid glutamine. Although these effects are not dramatic, conformity to the N-end rule is complex depending on additional factors such as inherent structure of the N-terminal region and presence of lysine residues for ubiquitin modification (Varshavsky 2011). Thus, the effects of N -terminal residues may be much more significant for proteins other than GFP.
In addition, it is well documented that expression levels in C. elegans are often strongly influenced by 3′ UTR sequences especially in germline tissue (Merritt et al. 2008). We replaced the ttb-2 3′ UTR with multiple widely used 3′ UTRs as well as the native mec-4 3′ UTR and the neuronal unc-10 3′ UTR. The effect on GFP levels ranged over 10-fold. let-858 was the most and unc-10 the least efficacious 3’ sequence. Note that we used a short unc-54 3′ UTR rather than the traditional longer sequence that contains the aex-5 promoter and often yields posterior intestinal background expression (Silva-García et al. 2019). These experiments highlight the robust influence 3′ UTRs have on expression levels. Which 3′ UTRs are most favorable is likely to be cell-type specific, so the same UTRs may not be the most robust in other cell types.
To assess if the effects are additive we created a transgene that incorporated the most effective trans-splicing and protein stability signals and the most optimal 3’ UTR. Disappointingly the multi-mutant construct expressed less strongly than the individual modified promoters, indicating that the elements interact with each other in complex ways to determine the overall expression level. Since this result was unexpected, we quantified the expression levels of 4 independently isolated identical insertions of the multi-mutant construct, and all behaved very similarly. This supports our prior experience that the jsTi1453 landing site is not significantly influenced by epigenetic factors under standard laboratory conditions.
Finally, we manipulated the mec-4 promoter in a fashion that is unlikely to be easily performed for most promoters. Extensive analysis of the mec-3, mec-4 and mec-7 promoters as well as other mec-3/unc-86 regulated genes (Xue et al. 1992; Duggan et al. 1998; Zhang et al. 2002) has defined a consensus binding site for the critical UNC-86/MEC-3 transcription factor heterodimer [CATN(3-4)AAATGCAT]. The mec-4 promoter is known to contain one such sequence; CATtatAAATGTAT. We inserted an additional binding site 100 bp upstream of the known binding site by introducing 27 bp that contain CATaagAAATGTAT – an identical sequence to the native binding site in the mec-4 promoter at the critical bases (capitals). Introduction of this sequence increased expression over two-fold compared to the native promoter and was the most potent of the manipulations performed.
While our studies identify some modifications that can be introduced into transgenic constructs to increase expression, they do not define a clear set of rules that can be implemented to insure high expression. Nevertheless, the simplicity of RMCE integration should make altering transgenic constructs a more realistic option to attempt before resorting to creating multicopy integrated transgenes.
Methods
Request a detailed protocolMethods
C. elegans was maintained on NGM agar plates spotted with OP50 at 22.5°C or at 25°C during the RMCE protocol.
RMCE transgenesis
Inserts were cloned into pLF3FShC (Addgene # 153083; Nonet, 2020) and injected at ~50 ng/µl into jsTi1453; bqSi711 young adults. Integrants were identified and isolated as described in detail in Nonet (2020). Single copy insertions were outcrossed to jsTi1453; him-8(e1489) to create jsTi1453 jsSi#; him-8(e1489) strains.
Microscopy
For quantification of GFP signals, homozygous L4 hermaphrodite animals were mounted on 2% agar pads in a 2 µl drop of 1mM levamisole in phosphate buffered saline and imaged on an Olympus (Center Valley, PA) BX-60 microscope equipped with a Qimaging (Surrey, BC Canada) Retiga EXi monochrome CCD camera, a Lumencor AURA LED light source, Semrock (Rochester, NY) GFP-3035B and mCherry-A-000 filter sets, and a Tofra (Palo Alto, CA) focus drive, run using micro-manager 2.0ß software (Edelstein et al. 2014) using a 40X air lens at 20% LED power with 200 ms exposures. PLM soma and ALM soma signals were quantified using the FIJI version of ImageJ software (Schindelin et al. 2012) as described in Nonet (2020).
Plasmid constructions
Modified versions of the NM3732 pLF3FShC mec-4p GFP-C1 tbb-2 plasmid were performed by SapI Golden Gate (GG) assembly inserting modified components from DR274 insert constructs as outlined below. DR274 entry vectors were created by inserting PCR fragments into the vectors using a BsaI GG reaction. Assembly reactions were performed as described in Nonet (2020).
The following were used:
pDD372 GFP-C1 (Dickinson et al. 2018)
NMp3469 DR274 FP-BsaI (Nonet, 2020)
NMp3643 pLF3FShC (Nonet, 2020)
NMp3694 DR274 AAG GTA tbb-2 3′ UTR (Nonet, 2020)
NMp3702 DR274 AAG GTA-BsaI (Nonet, 2020)
NM93732 pLF3FShC mec-4p GFP-C1 tbb-2 3’ UTR (Nonet, 2020)
NMp3751 DR274 AAG GTA act-4 3′ UTR (Nonet, 2020)
NMp3758 DR274 AAG GTA mec-4 3′ UTR
The mec-4 3’ UTR was amplified from N2 genomic DNA using oligonucleotides NMo6654/6655 and inserted into NMp3702.
NMp3759 DR274 AAG GTA unc-10 3′ UTR
The unc-10 3’ UTR was amplified from N2 genomic DNA using oligonucleotides NMo6656/6657 and inserted into NMp3702.
NMp3760 DR274 AAG GTA unc-54 3′ UTR
The unc-54 3’ UTR was amplified from N2 genomic DNA using oligonucleotides NMo6658/6659 and inserted into NMp3702.
NMp3761 DR274 FP (MV)GFP C1
GFP-C1 was amplified from pDD372 using NMo6660/6662 and inserted into NMp3469.
NMp3762 DR274 FP (MQ)GFP C1
GFP-C1 was amplified from pDD372 using NMo6661/6662 and inserted into NMp3469.
NMp3763 DR274 TGG ATG mec-4p
The mec-4 promoter was amplified from N2 genomic DNA using oligonucleotides NMo6663/6664 and inserted into NMp3698.
NMp3764 DR274 TGG ATG mec-4p wKr
The mec-4 promoter was amplified from N2 genomic DNA using oligonucleotides NMo6663/6665 and inserted into NMp3698.
NMp3765 DR274 TGG ATG mec-4p TS
The mec-4 promoter was amplified from N2 genomic DNA using oligonucleotides NMo6663/6667 and inserted into NMp3698.
NMp3766 DR274 AAG GTA let-858 3′ UTR
The let-858 3’ UTR was amplified from N2 genomic DNA using oligonucleotides NMo6656/6657and inserted into NMp3702.
NMp3778 DR274 TGG ATG mec-4p TS(-)
The mec-4 promoter was amplified from N2 genomic DNA using oligonucleotides NMo6663/6666 and inserted into NMp3698.
NMp3779 pLF3FShC mec-4p (MV)GFP-C1
The mec-4 promoter from NMp3763, GFP-C1(MV) from NMp3761, and the tbb-2 3’ UTR from NMp3694 were co-assembled into NMp3643.
NMp3780 pLF3FShC mec-4p (MQ)GFP-C1
The mec-4 promoter from NMp3763, GFP-C1(MQ) from NMp3762, and the tbb-2 3’ UTR from NMp3694 were co-assembled into NMp3643.
NMp3781 pLF3FShC mec-4p GFP-C1 act-4 3′
The mec-4 promoter from NMp3763, GFP-C1 from pDD372, and the act-4 3’ UTR from NMp3751 were co-assembled into NMp3643.
NMp3782 pLF3FShC mec-4p GFP-C1 let-858 3′
The mec-4 promoter from NMp3763, GFP-C1 from pDD372, and the let-858 3’ UTR from NMp3766 were co-assembled into NMp3643.
NMp3785 pLF3FShC mec-4p GFP-C1 mec-4 3′
The mec-4 promoter from NMp3763, GFP-C1 from pDD372, and the mec-4 3’ UTR from NMp3758 were co-assembled into NMp3643.
NMp3786 pLF3FShC mec-4p GFP-C1 unc-10 3′
The mec-4 promoter from NMp3763, GFP-C1 from pDD372, and the unc-10 3’ UTR from NMp3759 were co-assembled into NMp3643.
NMp3787 pLF3FShC mec-4p GFP-C1 unc-54 3′
The mec-4 promoter from NMp3763, GFP-C1 from pDD372, and the unc-54 3’ UTR from NMp3760 were co-assembled into NMp364.
NMp3788 pLF3FShC mec-4p wKr GFP-C1
The mec-4 worm Kozak replacement promoter from NMp3764, GFP-C1 from pDD372, and the tbb-2 3’ UTR from NMp3694 were co-assembled into NMp3643
NMp3789 pLF3FShC mec-4p TS GFP-C1
The mec-4 optimized trans-splice promoter from NMp3765, GFP-C1 from pDD372, and the tbb-2 3’ UTR from NMp3694 were co-assembled into NMp3643.
NMp3790 pLF3FShC-mec-4p TS(-) GFP-C1
The mec-4 trans-splice signal lesioned promoter from NMp3778, GFP-C1 from pDD372, and the tbb-2 3’ UTR from NMp3694 were co-assembled into NMp3643.
NMp3824 pLF3FShC mec-4(+u86m3)p GFP-C1
MNp3732 was amplified with oligonucleotides NMo6742/6743, kinased and religated. NMp4000 DR274 TGG ATG mec-4p TS wK+
The mec-4 promoter was amplified from NMp3779 and using oligonucleotides NMo6663/7007 and inserted into NMp3698.
NMp4001 DR274 TGG ATG mec-4p wK+
The mec-4 promoter was amplified from NMp3779 and using oligonucleotides NMo6663/7028 and inserted into NMp3698.
NMp4009 pLF3FShC mec-4p TS wK+ GFP-C1
The optimized mec-4 promoter from NMp4000, GFP-C1 from pDD372, and the tbb-2 3’ UTR from NMp3694 were co-assembled into NMp3643.
NMp4010 pLF3FShC mec-4p wK+ GFP-C1
The optimized Kozak site mec-4 promoter from NMp4001, GFP-C1 from pDD372, and the tbb-2 3’ UTR from NMp3694 were co-assembled into NMp3643.
NMp4020 pLF3FShC mec-4p TS wK+ (MV)GFP-C1 let-858
The optimized mec-4 promoter from NMp4000, (MV)GFP-C1 from NMp3761, and the let-858 3’ UTR from NMp3782 were co-assembled into NMp3643.
Oligonucleotides
| NMo number | Sequence |
| 6652 | ttGGTCTCAgAAGTGAattttcaaattttaaatactgaatatttg |
| 6653 | gcGGTCTCTcTACccaagcgaggacaattct |
| 6654 | ttGGTCTCAgAAGTGAatttgttttttcttgttttaaagtt |
| 6655 | gcGGTCTCTcTACgcagcttacagtatctttgtatt |
| 6656 | ttGGTCTCAgAAGTAAcaaatttcatatgtttttgtttgtt |
| 6657 | gcGGTCTCTcTACcattctccgttttctattgagt |
| 6658 | ttGGTCTCAgAAGTGAAGCTCCGCATCGG |
| 6659 | gcGGTCTCTcTACgtcataaactgaaacgtaacatatg |
| 6660 | ttGGTCTCAgATGGTCAGTAAAGGAGAAGAATTGTTCACT |
| 6661 | ttGGTCTCAgATGCAGAGTAAAGGAGAAGAATTGTTCACT |
| 6662 | gcGGTCTCTcCTTGTAGAGCTCGTCCATT |
| 6663 | ttGGTCTCAgTGGggttccggagcagttc |
| 6664 | ttGGTCTCTtCATtctataacttgatagcgataa |
| 6665 | ttGGTCTCTtCATttttataacttgatagcgataaaaaaaatagc |
| 6666 | ttGGTCTCTtCATtggtggacttgatagcgataaaaaaaatagc |
| 6667 | ttGGTCTCTtCATtctgaaacttgatagcgataaaaaaaatagc |
| 6742 | GAAATGTATAGAATACCAGTGCCTGGTGTTTGAGATGTTCTG |
| 6743 | TTATGATCCATTTCAACACACTTTCATGGATCTTATCTTGC |
| 7007 | TTGGTCTCTTCATTTTTCTGAAACTTGATAGCGAT |
| 7028 | TTGGTCTCTTCATTTTtctataacttgatagcgataaa |
Reagents
Worm Strains
| NM strain | Genotype | Source |
| NM5161 | jsTi1453 I; bqSi711 IV | Nonet, 2020; CGC |
| NM5187 | jsTi1453 I; him-8(e1489) IV | Nonet, 2020; CGC |
| NM5209 | jsTi1453 jsSi1514 [mec-4p GFP-C1 tbb-2 3’] I; him-8(e1489) IV | Nonet, 2020; CGC |
| NM5285 | jsTi1453 jsSi1556 [mec-4p (MV)GFP-C1 tbb-2 3’] I; him-8(e1489) IV | This work |
| NM5286 | jsTi1453 jsSi1557 [mec-4p (MQ)GFP-C1 tbb-2 3’] I; him-8(e1489) IV | This work |
| NM5287 | jsTi1453 jsSi1558 [mec-4p GFP-C1 unc-10 3’] I; him-8(e1489) IV | This work |
| NM5288 | jsTi1453 jsSi1559 [mec-4p GFP-C1 act-4 3’] I; him-8(e1489) IV | This work |
| NM5289 | jsTi1453 jsSi1561 [mec-4p GFP-C1 mec-4 3’] I; him-8(e1489) IV | This work |
| NM5328 | jsTi1453 jsSi1562 [mec-4p TS(-) GFP-C1 tbb-2 3’] I; him-8(e1489) IV | This work |
| NM5329 | jsTi1453 jsSi1563 [mec-4p TS GFP-C1 tbb-2 3’] I; him-8(e1489) IV | This work |
| NM5330 | jsTi1453 jsSi1564 [mec-4p wKr GFP-C1 tbb-2 3’] I; him-8(e1489) IV | This work |
| NM5331 | jsTi1453 jsSi1566 [mec-4p GFP-C1 unc-54 3’] I; him-8(e1489) IV | This work |
| NM5332 | jsTi1453 jsSi1573 [mec-4p GFP-C1 let-858 3’] I; him-8(e1489) IV | This work |
| NM5333 | jsTi1453 jsSi1573 [mec-4(XB)p GFP-C1 tbb-2 3’] I; him-8(e1489) IV | This work |
| NM5436 | jsTi1453 jsSi1636 [mec-4p wK+ GFP-C1 mec-4 3’] I; him-8(e1489) IV | This work |
| NM5438 | jsTi1453 jsSi1637 [mec-4p TS wK+ GFP-C1 mec-4 3’] I; him-8(e1489) IV | This work |
| NM5441 | jsTi1453 jsSi1642 [mec-4p TS wK+ (MV)GFP-C1 let-858 3’] I; bqSi71 IV | This work |
| NM5450 | jsTi1453 jsSi1650 [mec-4p TS wK+ (MV)GFP-C1 let-858 3’] I; bqSi711 IV | This work |
| NM5451 | jsTi1453 jsSi1651 [mec-4p TS wK+ (MV)GFP-C1 let-858 3’] I; bqSi711 IV | This work |
| NM5452 | jsTi1453 jsSi1652 [mec-4p TS wK+ (MV)GFP-C1 let-858 3’] I; bqSi711 IV | This work |
Reagents
Plasmids and worm strains are available by request from MLN and will be submitted to Addgene and the Caenorhabditis Genetics Center if demand levels warrant it.
References
Funding
Department of neuroscience, WUMS.
Reviewed By
Eric LambieHistory
Received: April 21, 2021Revision received: May 10, 2021
Accepted: May 10, 2021
Published: May 12, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Dour, S; Nonet, M (2021). Optimizing expression of a single copy transgene in C. elegans. microPublication Biology. 10.17912/micropub.biology.000394.Download: RIS BibTeX