IBMC - Instituto de Biologia Molecular e Celular, Universidade do Porto, Porto, Portugal
Abstract
Cilia are microtubule-based organelles that carry out a wide range of critical functions throughout the development of higher animals. Regardless of their type, all cilia rely on a motor-driven, bidirectional transport system known as intraflagellar transport (IFT). Of the many components of the IFT machinery, IFT20 is one of the smallest subunits. Nevertheless, IFT20 has been shown to play critical roles in the assembly of several types of mammalian cilia. Here we show that the IFT20 homolog in Caenorhabditis elegans, IFT-20, is also important for correct cilium assembly in sensory neurons. Strikingly, however, we find that IFT-20-deficient animals are able to assemble short, vestigial cilia. In spite of this, we show that practically all IFT-20-deficient animals fail to respond to environmental cues that are normally detected by cilia to modulate their behavior. Altogether, our results indicate that IFT-20 is critical for both the correct assembly and function of cilia in C. elegans.
Description
A high diversity of cilium types have evolved to carry out a multitude of sensorial and motility functions in animals. It is therefore not surprising that defects in cilium structure and function have been associated with various severe multi-organ disorders, commonly known as ciliopathies (Sreekumar and Norris 2019). Regardless of their type, cilium assembly relies on bidirectional motor-driven transport known as intraflagellar transport (IFT; Prevo et al. 2017). Anterograde IFT driven by kinesin-2 transports different cargos in IFT trains along axonemal microtubules from the ciliary base to the tip of the cilium. Upon reaching the tip, these IFT trains are dismounted, rearranged and prepared for transport in the opposite direction back to the ciliary base by the dynein-2 motor complex. IFT trains are composed of at least 16 IFT-B subunits and 6 IFT-A subunits (Prevo et al. 2017). IFT20 is part of the IFT-B complex and has been shown to be of critical importance for ciliogenesis in human and mouse models (Follit et al. 2006; Jonassen et al. 2008; Katoh et al. 2017; Lim et al. 2020).
In C. elegans, IFT-20 is encoded by the Y110A7A.20 locus on chromosome I. Y110A7A.20 was identified in 2004 as a locus containing a X-box sequence (Fan et al. 2004; Blacque et al. 2005; Efimenko et al. 2005) under the control of DAF-19, a RFX-type transcription factor that regulates ciliary gene expression in ciliated sensory neurons (Swoboda et al. 2000) (the only ciliated cells in C. elegans). Expression of a GFP reporter under the control of the ift-20 promoter revealed that IFT-20 expression is restricted to sensory neurons, similarly to other IFT components (Blacque et al. 2005). In fact, disruption of daf-19 resulted in the almost complete loss of expression of this GFP reporter (Blacque et al. 2005), confirming that DAF-19 drives IFT-20 expression most likely by recognizing the X-box at position -59 of the ift-20 promoter (Blacque et al. 2005; Efimenko et al. 2005). Later, Ou and colleagues constructed a vector for IFT-20::GFP expression, and found that the C. elegans IFT20 homolog is also recruited to cilia where it undergoes IFT with similar kinetics to other IFT subunits (Ou et al. 2007). However, in contrast to its homolog in mammalian cells, IFT-20 was not detectable at the Golgi and does not seem to interact with the C. elegans homolog of GMAP210 (Broekhuis et al. 2013), which anchors IFT20 to the Golgi in mammalian cells (Follit et al. 2008).
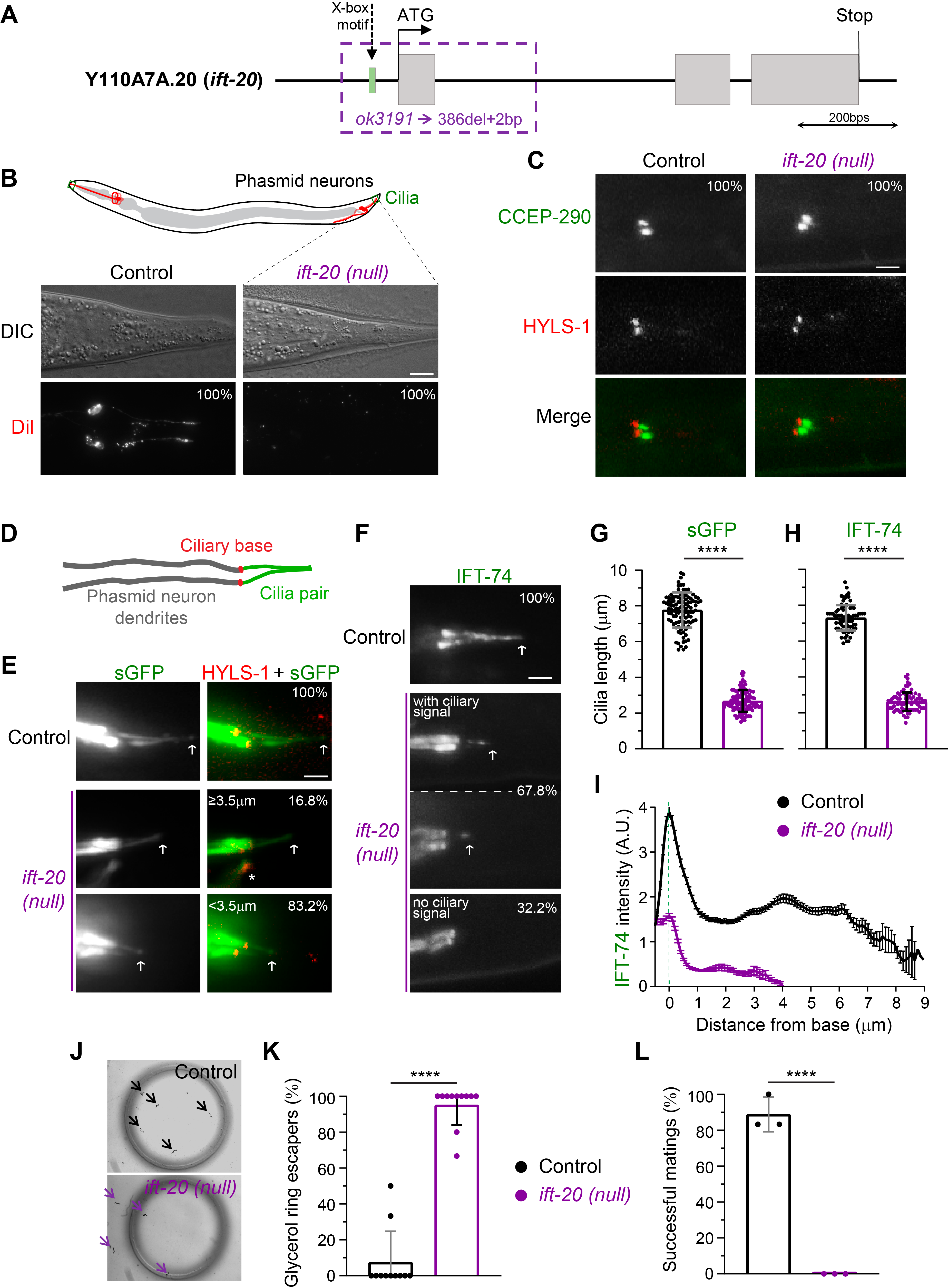
In spite of these early analyses of IFT-20 expression and localization, the extent to which IFT-20 contributes to ciliogenesis in C. elegans remains unknown. To dissect the importance of IFT-20, we obtained and mapped a null deletion allele (ok3191) of the ift-20 locus (Figure 1A). This allele carries a deletion of 386 bps that includes part of the 5′ UTR, the X-box motif that controls ift-20 expression, the start codon, and all of the 1st exon (sequencing data was submitted to WormBase). Because the expression of ift-20 was previously shown to be under the control of the DAF-19 transcription factor, and since ok3191 lacks the complete first exon, we expect that this allele results in the complete loss of IFT-20. Thus, we refer to the ift-20 (ok3191) allele as an ift-20 null.
We started our analysis of ift-20 null worms by performing the classic dye-filling assay to test whether cilia are able to form normally in the absence of IFT-20. This assay takes advantage of the fact that the lipophilic, fluorescent DiI dye is specifically incorporated into a subset of ciliated sensory neurons through their cilia (if these are present and in contact with the outside environment; Extended Data Figure 1A). In contrast to the wild-type controls that incorporated the dye, none of the ift-20 null worms had DiI signal in their neurons (Figure 1B; Extended Data Figure 1B). To confirm that this dye-filling phenotype results from ciliary defects rather than abnormal dendritic morphology/extension, we expressed cytoplasmic GFP in sensory neurons (driven by the osm-6/ift-52 promoter) to analyze their morphology. We found no significant differences in the length and morphology of sensory neurons (Extended Data Figure 1C), which, together with our dye-filling results, suggest that IFT-20 loss leads to defects in sensory cilia.
Using established markers for the cilium base (HYLS-1) and the ciliary transition zone (CCEP-290) (Schouteden et al. 2015), we found that ift-20 null worms still positioned their basal body normally at the extremity of the dendrite in phasmid neurons and were able to form at least the CCEP-290 module of the ciliary transition zone (Figure 1C). We then took advantage of the incorporation of the soluble/cytoplasmic GFP into cilia of phasmid sensory neurons to visualize cilia and measure their length in ift-20 null worms. Strikingly, we found that severely shortened/vestigial cilia were still able to form in phasmid neurons of ift-20 null animals (Figure 1D,E,G). This suggests that IFT-20 loss leads to a severe defect in ciliary axoneme assembly but does not completely block ciliogenesis in C. elegans.
In order to directly visualize IFT particles in cilia of ift-20 null animals, we analyzed the distribution of IFT-74::GFP (Yi et al. 2017), another subunit of the IFT-B complex. IFT-74::GFP was readily detectable in dendrites of amphid and phasmid neurons, which allowed us to further confirm the normal development and morphology of these neurons in ift-20 null worms (Extended Data Figure 1D). In controls, IFT-74::GFP was strongly recruited to cilia, labeling the complete axoneme (Figure 1F,H). In contrast, IFT-74::GFP-labeled cilia were severely shortened in the majority of ift-20 null phasmid neurons (67.8%; Figure 1F,H), consistent with our observations using soluble GFP-labeled cilia. Interestingly, however, no IFT-74::GFP-labeled cilia were detectable in the remaining fraction of phasmid neurons (32.2%; Figure 1F), suggesting that IFT-20 is also required for IFT-74 incorporation into cilia. To further confirm this possibility, we quantified the distribution of any detectable IFT-74::GFP signal in phasmid cilia of ift-20 null worms. We found that the signal intensity of IFT-74::GFP recruited to the ciliary base and inside cilia of phasmid sensory neurons was indeed strongly reduced in ift-20 null worms (Figure 1I). These results reveal that, in addition to being important for cilia assembly in C. elegans, IFT-20 plays a role in the ciliary recruitment and entry of IFT subunits.
The fact that short cilia could still form in phasmid sensory neurons of ift-20 null worms was somewhat unexpected given the critical role of IFT20 for the initiation of ciliogenesis in most mammalian models (Follit et al. 2006; Jonassen et al. 2008; Katoh et al. 2017). Interestingly, however, we note that very short flagella with abnormal axonemes (but still motile) can form in sperm of mice with male germline-specific disruption of IFT20 (Zhang et al. 2016).
To assess the degree of functionality of the IFT-20-deficient vestigial cilia, we performed osmotic and mating assays. When performing the osmotic avoidance assay, virtually all ift-20 null worms readily crossed the glycerol rings that wild-type worms avoided (Figure 1J,K). This suggests that IFT-20-deficient cilia fail to sense normally repulsive concentrations of glycerol. In agreement with defective ciliary sensory functions, all ift-20 null male worms failed to mate (Figure 1L), suggesting that IFT-20-deficient cilia in male tails were unable to detect wild-type hermaphrodites for mating. Together, the results of these behavioral assays strongly suggest that IFT-20 loss completely impairs cilia-dependent functions in C. elegans. Although we did not analyze the morphology and IFT of all types of sensory cilia in the ift-20 null, our analysis of cilia-dependent behavior is consistent with the cilium assembly defects that we observed in ift-20 null phasmid sensory neurons.
Altogether, our results show that IFT-20 is required for ciliogenesis and cilia sensory functions in C. elegans. Our data also suggest that IFT-20 is important for the robust recruitment and entry of IFT subunits into vestigial cilia that assemble in ift-20 null animals.
Methods
Request a detailed protocolCaenorhabditis elegans maintenance and strain generation
C. elegans strains were maintained on standard nematode growth medium (NGM) plates seeded with Escherichia coli OP50 bacteria at 20 °C, and crossed using standard procedures. Mutant genotyping was performed by standard PCR. C. elegans strains used in this study are listed in Table 1.
Fluorescence imaging
Young adult hermaphrodite worms were immobilized for imaging using 10 mM Levamisol and were placed on a microscope slide with a 5% agarose pad. Imaging of worms and their cilia was carried out over at least 3 independent experiments using an Axio Observer microscope (Zeiss) controlled by ZEN software (Zeiss), equipped with a 63x 1.46 NA objective lens and an Orca Flash 4.0 camera (Hamamatsu). Z-stacks were acquired with 0.4 µm between each z-section for imaging cilia. Z-stack images were processed and analyzed with Fiji software (Image J version 2.0.0-rc-56/1.52 p). Fluorescence signals of cilium-incorporated soluble GFP (with mCherry::HYLS-1), and IFT-74::GFP were used to measure the length of cilia in phasmid neurons (starting at the maximum signal at the base). The IFT-74::GFP signal intensity of each pixel was measured from the base to the tip of each cilium to generate an averaged profile of IFT-74 distribution along cilia.
Dye-Filling
Worms from each strain were collected from a confluent but not starved plate and washed in M9 buffer. They were then incubated in 500 µL of DiI (1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindocarbocyanine Perchlorate) solution at 0.625 µg/ml for 1 hr at room temperature in the dark, with occasional flipping of the tubes. After washing worms in M9 buffer, these were placed on a NGM seeded plate for 1-3 hr at 20 °C, to reduce the background of ingested dye. The neuronal uptake of dye was then examined using the Axio Observer microscope, as described above.
Mating Assay
Mating assays were performed as described in (Hodgkin 1983) for quantitative mating efficiency tests. First, 6x young males of the strain to be tested were separated 24 hr prior to the experiment on a 5 cm NGM seeded plate at 20 °C. These males were then placed together with 6x L4 wild-type hermaphrodites on a mating plate (i.e. a 5 cm NGM agar plate with an ~1 cm spot of OP50 seeded at the center of the plate). The worms were then allowed to mate for 24 hr at 20 °C, after which the hermaphrodites were isolated onto a new 5 cm NGM seeded plate and kept at 20 °C for 24 hr to lay eggs. After this period, hermaphrodites were removed and plates with eggs were kept at 25 °C. Two days after, the number of total progeny and the number of male progeny on the plate were counted. In order to obtain enough males to be used in these assays, both strains used for mating contained a him-8 mutation, which leads to an increase in mis-segregation of the X chromosome during meiosis I (Phillips et al. 2005).
Osmotic Avoidance Assay
Osmotic avoidance assays were performed on NGM non-seeded plates at 20 °C, following the guidelines of (Sanders et al. 2015). Each repeat was carried out using five young adult hermaphrodite worms of each strain isolated before the experiment. Worms were placed inside of a glycerol ring, with a diameter of approximately 1 cm, freshly prepared with a tube dipped in a 59% glycerol solution. Worm behavior was immediately monitored for 5 min using a camera (controlled by the Micro-Manager 1.4 software) to determine whether worms avoided crossing the glycerol ring or not. Worms that left the ring or stayed in contact with its glycerol border for more than 20 sec were classified as escapers. Worms that failed to explore the area within the timeframe of the experiment were excluded.
Data analysis and statistics
Statistical analyses of datasets were performed using GraphPad Prism software. The two-tailed student T test was used to analyze the data in the graphs of Figure 1. Differences were considered significant at P values below 5% (*P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001).
Reagents
Table 1: List of C. elegans strains used in this work.
| Strain | Genotype | Short description | Source/Ref. |
| N2 | wild-type | ancestral N2 Bristol | CGC |
| RB2353 | ift-20(ok3191)I | ift-20(null) | OMRF Knockout Project/ CGC |
| GOU2362 | ift-74(cas499[ift-74::gfp])II | IFT-74::GFP knock-in | Dr. Guangshuo Ou/CGC (Yi et al. 2017) |
| DAM456 | vieSi12[pAD373; Pccep-290::gfp::ccep-290cDNA; cb unc-119(+)]II; vieSi16[pAD390; Phyls-1::mCherry::hyls-1; cb unc-119(+)]IV | GFP::CCEP-290 (Mos transposase-mediated single-copy insertion (MosSCI)) +
mCherry::HYLS-1 (MosSCI) |
Dr. Alexander Dammermann (Schouteden et al.2015) |
| AND16 | ift-20(ok3191)I | ift-20(null) outcrossed 6x | This study, made from RB2353 |
| AND56 | him-8(e1489)IV | him-8 mutant for matting assay | This study, made from OE3002 |
| AND67 | ift-20(ok3191)I; him-8(e1489)IV | ift-20(null) + him-8 mutant for matting assay | This study |
| AND185 | ift-20(ok3191)I; ift-74(cas499[ift-74::gfp])II | ift-20(null) +
IFT-74::GFP |
This study |
| AND196 | ift-20(ok3191)I; vieSi12[pAD373; Pccep-290::gfp::ccep-290cDNA; cb unc-119(+)]II; vieSi16[pAD390; Phyls-1::mCherry::hyls-1; cb unc-119(+)]IV | ift-20(null) +
GFP::CCEP-290 (MosSCI) + mCherry::HYLS-1 (MosSCI) |
This study |
| AND226 | vieSi16[pAD390; Phyls-1::mCherry::hyls-1; cb unc-119(+)]IV; lqIs2[Posm-6::GFP; lin-15(+)]X | mCherry::HYLS-1 (MosSCI) +
Posm-6::GFP |
This study, made from LE309 |
| AND227 | ift-20(ok3191)I; vieSi16[pAD390; Phyls-1::mCherry::hyls-1; cb unc-119(+)]IV; lqIs2[Posm-6::GFP; lin-15(+)]X | ift-20(null) +
mCherry::HYLS-1 (MosSCI) + Posm-6::GFP |
This study, made from LE309 |
Acknowledgments
We thank Dr. Alexander Dammermann and Dr. Guangshuo Ou for providing strains. We also thank the C. elegans Gene Knockout Project at the Oklahoma Medical Research Foundation for generating the original ift-20 mutant strain. Some strains were obtained from the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). We are also very grateful to Dr. Reto Gassmann (IBMC/i3S) for critical reading of the manuscript, and the Gassmann and the Carvalho labs at i3S for technical help with experiments.
Extended Data
Dantas, T. J. (2021). Extended Data Figure 1: Morphology of amphid and phasmid neurons in IFT-20-deficient worms (Version 1.0). CaltechDATA. 10.22002/D1.1966
References
Funding
This work was financed by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through the Fundação para a Ciência e a Tecnologia (FCT) / Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project POCI-01-0145-FEDER-029471 (PTDC/BIA-BID/29471/2017) to TJD. DRMR received a predoctoral fellowship from FCT (SFRH/BD/143985/2019). CMA and TJD salaries were also supported by the FCT: CEECIND/01985/2018 and CEECIND/00771/2017, respectively.
Reviewed By
AnonymousHistory
Received: February 22, 2021Revision received: May 4, 2021
Accepted: May 6, 2021
Published: May 11, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
De-Castro, ARG; Quintas-Gonçalves, J; Silva-Ribeiro, T; Rodrigues, DRM; De-Castro, MJG; Abreu, CM; Dantas, TJ (2021). The IFT20 homolog in Caenorhabditis elegans is required for ciliogenesis and cilia-mediated behavior. microPublication Biology. 10.17912/micropub.biology.000396.Download: RIS BibTeX