Abstract
Drosophila melanogaster vinegar flies have two olfactory organs: the antenna and maxillary palp. Olfactory neurons in these tissues respond to odorants via odorant receptors. Insect odorant receptors are heterotetramers of two proteins: an odorant binding OrX subunit and an Odorant Receptor Co-Receptor (Orco). Mutation of Orco disrupts odorant receptor formation, and abolishes olfactory responses. Some antennal olfactory neurons in Orco mutants have been previously shown to degenerate. Here, we examine if maxillary palp olfactory neurons also degenerate in Orco mutants. We find degeneration occurs both more broadly and more rapidly in Orco mutant maxillary palp olfactory neurons than reported for antennae, with ~60% of all mutant olfactory neurons absent in maxillary palps by 7 days post eclosion. Interestingly, the subset of Orco mutant olfactory neurons that express the Or42a receptor appear resistant to degeneration. These results suggest the maxillary palp might be a suitable model for examining the molecular mechanisms underlying neurodegeneration in sensory neurons.
Description
The odorant receptor co-receptor Orco forms ligand-gated ion channels with odorant receptors (ORs) tuned to specific odors; Orco is required for trafficking of these complexes to the dendrites of olfactory neurons (Larsson et. al., 2004). Mutation of the Orco gene not only abolishes olfactory neuron activity but has also revealed potential new roles for this gene in the development and maintenance of olfactory systems in a variety of insects. In ants and honeybees, Orco mutation leads to a loss of olfactory sensory neurons in the periphery, as well as severe defects in the morphology of the antennal lobe, the first olfactory processing center in the insect brain (Trible et al., 2017; Yan et al., 2017; Chen et al., 2021). These deficits are likely developmental as they are apparent at adult eclosion. Orco mutant hawkmoth males show more modest losses in antennal lobe volume associated with pheromone-sensing olfactory neurons (Fandino et al., 2019). In contrast, in Anopheles coluzzii and Aedes aegypti mosquitoes Orco mutation does not appear to lead to olfactory neuron loss or gross deficits in antennal lobe anatomy (though the latter has not been examined in depth) (Sun et al., 2020; DeGennaro et al., 2013).
In the vinegar fly Drosophila melanogaster, development of the adult olfactory system in Orco mutants appears normal (Larsson et. al., 2004). However, over time some populations of olfactory neurons in the antennae degenerate. This degeneration is initially evident in the olfactory neuron axons innervating the antennal lobe, which start to show signs of blebbing and retraction four to six days post eclosion (DPE) (Chiang et al., 2009). By 14 DPE, some but not all antennal olfactory neurons lose their cell bodies (Hueston et al., 2016). There is evidence that the cause of antennal olfactory neuron degeneration in the Orco mutant is due to a lack of neuronal activity (Chiang et al., 2009). Here, we investigated if neurodegeneration in Orco mutants also occurs in the Drosophila maxillary palp, a simpler olfactory organ with ~120 olfactory neurons.
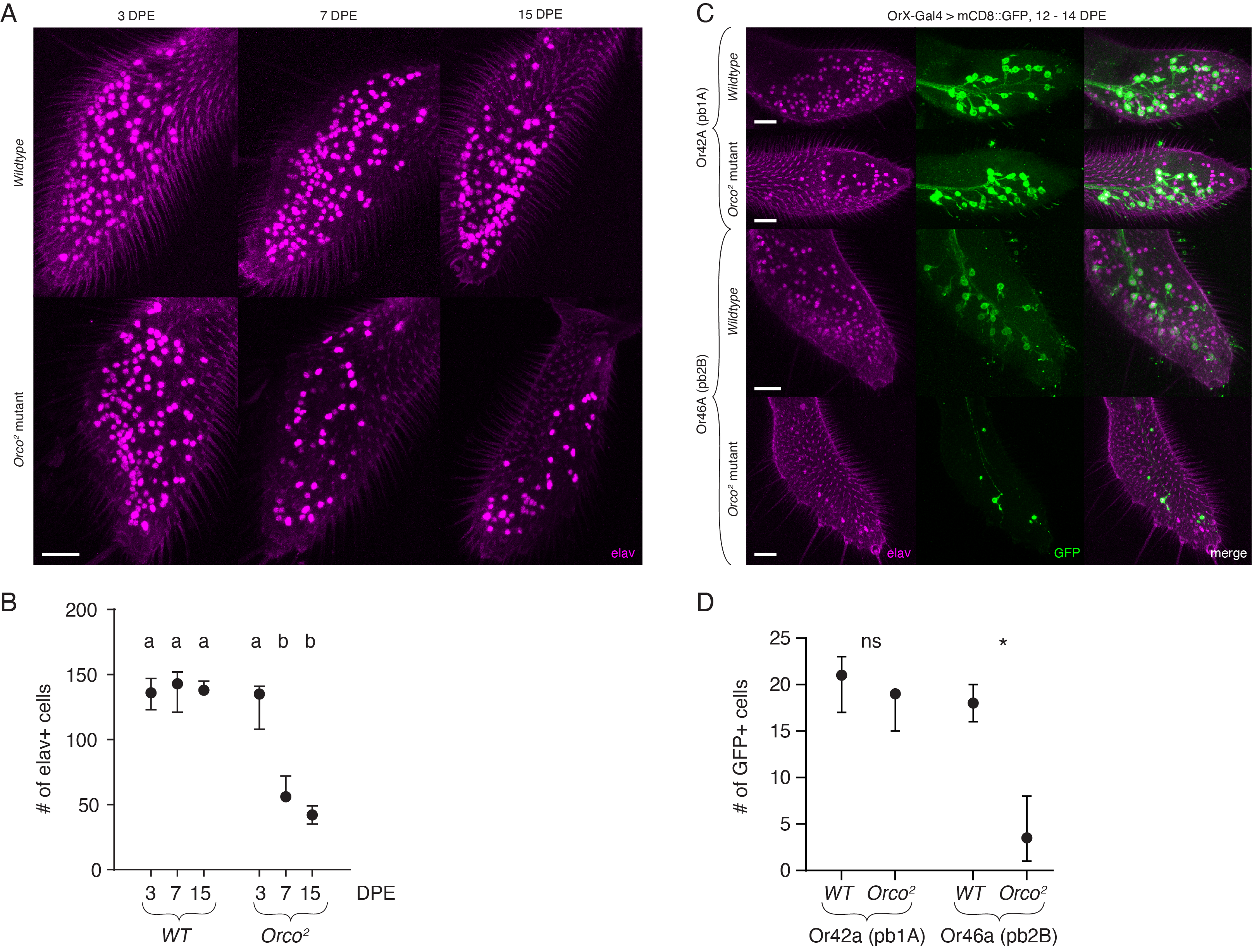
To examine the effects of the loss of Orco on maxillary palp olfactory neurons, we performed whole-mount antibody staining on maxillary palps using the pan-neuronal marker, elav (Robinow and White, 1991). This marker should label all ~120 olfactory neurons in the palps, as well as a small population (~20) of presumed mechanosensory neurons (Singh and Nayak, 1985). Neuronal cell death should result in a reduction in the number of elav+ cells. Potential alternative approaches could be to use DAPI staining or the TUNEL assay to visualize cell loss and DNA fragmentation associated with cell death, among others (Meehan et al., 2015). We compared anti-elav antibody staining in wildtype and Orco2 mutant flies at three timepoints: 3, 7, and 15 days post eclosion (DPE) (Fig 1A). In the wildtype, we found consistent neuronal cell counts across timepoints. The Orco2 mutant palps of 3-day-old flies were indistinguishable from wildtype (Fig 1A). However, by 15 DPE, extensive loss of cell bodies was detectable in the Orco2 mutants (Fig 1A). This neurodegeneration was evident at 7 DPE (middle column), suggesting that cell body loss in the maxillary palp occurs more quickly and potentially more broadly than in the antennae. Overall, Orco2 mutants lose 60 – 70% of their maxillary palp neurons. These results are quantified in Fig 1B. Both 7 and 15 DPE Orco2 mutants had significantly fewer elav+ cells compared to all wildtype timepoints as well as 3 DPE Orco2 mutants (p ≤ 0.0002) but were not statistically significantly different from each other (p = 0.4055) (one-way ANOVA with Tukey’s HSD; see figure legend for F statistic and exact p values for all pairwise comparisons). There was no difference between any of the wildtype conditions (p > 0.99), nor between the 3 DPE Orco2 mutant and any of the wildtype timepoints (p > 0.79).
We further examined if sub-populations of olfactory neurons in the maxillary palp might be resistant to degeneration. There are six different populations of olfactory neurons in the maxillary palp as defined by the odor-responsive OrX they express. Using available genetic reagents, we examined neuron degeneration in two of these populations in the Orco mutant background. We used the GAL4/UAS system (Brand and Perrimon, 1993) to GFP label the pb1A (Or42a-GAL4/UAS-mCD8:GFP) or pb2B (Or46a-GAL4/UAS-mCD8:GFP) olfactory neurons (Goldman et al., 2005) in wildtype or Orco2 mutant maxillary palps. We examined flies 12 – 14 DPE to allow sufficient time for degeneration. Surprisingly, we found that the pb1A (Or42a-expressing) olfactory neuron population was largely spared from degeneration (Fig 1C, quantified in Fig 1D). In contrast, the pb2B (Or46a-expressing) olfactory neurons were almost all missing by 14 DPE (Fig 1C, quantified in Fig 1D). Those pb2B cells that remained showed signs of neuronal process fragmentation and blebbing, consistent with previously reported antennal neuron degeneration (Chiang et al., 2009). Prior work has confirmed that both these populations express Orco (Grabe et al., 2016; Larsson et al., 2004; Task et al., 2020). Our results suggest that at least one sub-population of Orco mutant olfactory neurons are resistant to activity-dependent cell death and represents the first quantified example of a specific olfactory neuron population escaping degeneration. While pb1A cells are presumably non-functional due to a lack of Orco, future experiments will be required to confirm that this is indeed the case, especially in light of recent studies showing co-expression of odorant receptors with other, non-Orco-dependent chemoreceptors in the same cells (McLaughlin et al., 2021; Task et al., 2020; Younger and Herre et al., 2020). This could be achieved by genetically silencing pb1A activity, as has previously been done in the antenna (Chiang et al., 2009). Interestingly, Orco-dependent cell death in the antennae does not involve caspase-dependent pathways and cannot be rescued by expression of the pan-caspase inhibitor p35 (Chiang et al., 2009). It remains to be determined if Orco mutant neurons in the maxillary palp similarly engage caspase-independent molecular mechanisms.
The olfactory system of insects represents a convenient model for examining how a lack of induced activity might affect the health of a sensory neuron (MacDonald et al., 2006; Chiang et al., 2009; Kazama et al., 2011; Hueston et al., 2016). As shown here, the maxillary palp presents a potentially favorable system for studying neurodegeneration and activity-dependent neuronal maintenance. In contrast to the antenna, neuronal cell body loss in Orco mutant palps is rapid (within 6 or 7 days post eclosion vs. 14 days post eclosion in antennae). The palp is a simpler olfactory organ, and neuronal changes are easier to study and quantify by whole-mount staining in the palps. We present evidence that at least one genetically definable population of olfactory neurons appears resistant to degeneration. Future experiments could examine if any of the other four olfactory sub-populations in the maxillary palp also demonstrate such resistance. Given the extensive genetic tools available in the Drosophila model system, maxillary palp olfactory neurons might be amenable to genetic screens aimed at investigating the molecular mechanisms underlying activity-dependent sensory degeneration.
Methods
Request a detailed protocolFly husbandry and Drosophila genetics
Fly stocks were maintained at 20 – 25°C on standard cornmeal-agar food. Male and female flies used for experiments were 3 – 15 days old. Exact age for each experimental condition indicated in Figure 1. Experimental flies were homozygous for Orco2, while control flies were either w1118 wildtypes (Figure 1A-B), or heterozygous for Orco2 (Orco2/TM6b; Figure 1C-D). Full genotypes of stocks used in Figure 1C-D: Or42a-Gal4, 10XUAS-IVS-mCD8::GFP / CyO ; Orco2 / TM6b and Or46a-Gal4, 10XUAS-IVS-mCD8::GFP / CyO ; Orco2 / TM6b.
Immunohistochemistry
All immunostaining steps were done while rotating. Fly proboscises (labella and palps) were dissected in 1XPBS and fixed in 4% paraformaldehyde in PBT (1XPBS + 0.3% Triton X-100) for 15 minutes at room temperature. Tissue was washed three times for 15 minutes each at room temperature in PBT, then blocked for at least 30 minutes at room temperature in blocking solution (PBT + 5% normal goat serum). Primary antibodies were added to fresh blocking solution, and tissue was incubated in this solution overnight at 4°C. On day two, tissue was washed three times for 15 minutes each at room temperature in PBT, then incubated in secondary antibodies in fresh block overnight at 4°C in the dark. On day three, tissue was washed three times for 15 minutes each at room temperature in the dark and mounted in SlowFade Gold (ThermoFisher S36936). Palps were dissected from labella on the slide before mounting. Primary antibodies were used at 1:100 concentration, secondary antibodies were used at 1:200 concentration. See Reagents for antibodies used.
Confocal imaging and analysis
Palps were imaged on a Zeiss LSM 700 confocal microscope equipped with a C-Apochromat 63x/1.2 water Korr M27 objective. Images were acquired at 512 x 512-pixel resolution with 0.58 µm z-step. For illustration purposes, confocal images were processed in Fiji/ImageJ to collapse Z-stacks into a single image using maximum intensity projection. Fiji was also used to adjust the gain in separate channels; no other image processing was performed on the confocal data. Elav+ and GFP+ cells were counted manually in Fiji using the Cell Counter plugin.
Statistics
All statistical analyses and plots were done in GraphPad Prism (version 8). For all analyses, significance level α = 0.05. In Figure 1A-B, one-way ANOVA with Tukey’s HSD post-hoc test was used to compare elav+ cell counts across both genotypes at the three timepoints. In Figure 1C-D, Mann Whitney U tests were used to compare GFP+ cell counts in the two genotypes within each neuron type.
Reagents
Drosophila melanogaster stocks used:
| Genotype | Source | Identifier |
| w[*]; P{w[+mC]=Or42a-GAL4.F}48.3B | Bloomington Drosophila Stock Center | BDSC: 9970; FlyBase: FBti0101811 |
| w[1118]; P(w[+mC]=Or46a-GAL4.G)32.1.y | Bloomington Drosophila Stock Center | BDSC: 23291; FlyBase: FBti0076800 |
| w[*]; P(y[+t7.7] w[+mC]=10XUAS-IVS-mCD8::GFP)attP40 | Bloomington Drosophila Stock Center | BDSC: 32186; FlyBase: FBti0131963 |
| Orco2 mutant: w[*]; TI(w[+m*]=TI)Orco[2] | Bloomington Drosophila Stock Center | BDSC: 23130; FlyBase: FBti0168777 |
| Wildtype: w1118 IsoD1 | Gift from Thomas R. Clandinin | Derived from FBal0018186 |
| Double Balancer: y,w; Pin/CyO; Dh/TM6B | Potter lab stock | Derived from FBal0013831, FBba0000025, FBti0004009, FBba0000057, FBal0016730 |
| Double Balancer: y,w; S/CyO; Pr/TM6B | Potter lab stock | Derived from FBal0015108, FBba0000025, FBal0013944, FBba0000057, FBal0016730 |
Antibodies used:
| Antibody | Source | Identifier |
| Rat anti-elav | DSHB | Cat# Rat-Elav-7E8A10; RRID: AB_528218 |
| Chicken anti-GFP | Aves Labs | Cat# GFP-1010; RRID: AB_2307313 |
| Goat anti-rat Cy3 | Jackson ImmunoResearch | Cat# 112-165-167; RRID: AB_2338251 |
| Goat anti-rat Alexa 647 | Jackson ImmunoResearch | Cat# 112-605-167; RRID: AB_2338404 |
| Goat anti-chicken Alexa 488 | Invitrogen | Cat# A11039; RRID: AB_142924 |
Acknowledgments
We thank Thomas R. Clandinin (Stanford) and the Bloomington Drosophila Stock Center for fly lines; the Center for Sensory Biology Imaging Facility (NIH P30DC005211) for use of the LSM700 confocal microscope; and members of the Potter lab for discussion.
References
Funding
This work was supported by grants to Christopher J. Potter from the Department of Defense (W81XWH-17-PRMRP) and from the National Institutes of Health (NIAID R01Al137078; NIDCD R01DC013070).
Reviewed By
Anonymous and Mani RamaswamiHistory
Received: March 8, 2021Revision received: May 5, 2021
Accepted: May 12, 2021
Published: May 14, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Task, D; Potter, CJ (2021). Rapid degeneration of Drosophila olfactory neurons in Orco mutant maxillary palps. microPublication Biology. 10.17912/micropub.biology.000398.Download: RIS BibTeX