School of Biological Sciences, The University of Adelaide, Australia
Department of Animal and Plant Sciences, The University of Sheffield, UK
School of Biological Sciences, The University of Adelaide, Australia
Abstract
Plant organ size control is an essential process of plant growth and development. The regulation of plant organ size involves a complicated network of genetic, molecular interactions, as well as the interplay of environmental factors. Here, we report a temperature-sensitive hypocotyl elongation EMS-generated mutant, hereby referred to as elongated hypocotyl under high-temperature (elh). The elongated hypocotyl phenotype was prominent when the elh seedlings were grown at high temperature, 28°C, but not under the growth temperature of 21°C. We observed significantly larger organ sizes in elh plants, including cotyledons, petals and seeds. In elh plants, the cell sizes in cotyledons and petals were significantly larger than wild type. By measuring the cell density and organ area of cotyledons, petals and mature dissected embryos, we found no differences in total cell numbers in any organ indicating that cell expansion rather than cell proliferation was perturbed in elh. elh plants produced leaves at a slower rate than wild type plants, suggesting that perturbing the balance between cell division and cell expansion is linked to the developmental rate at which leaves are produced.
Description
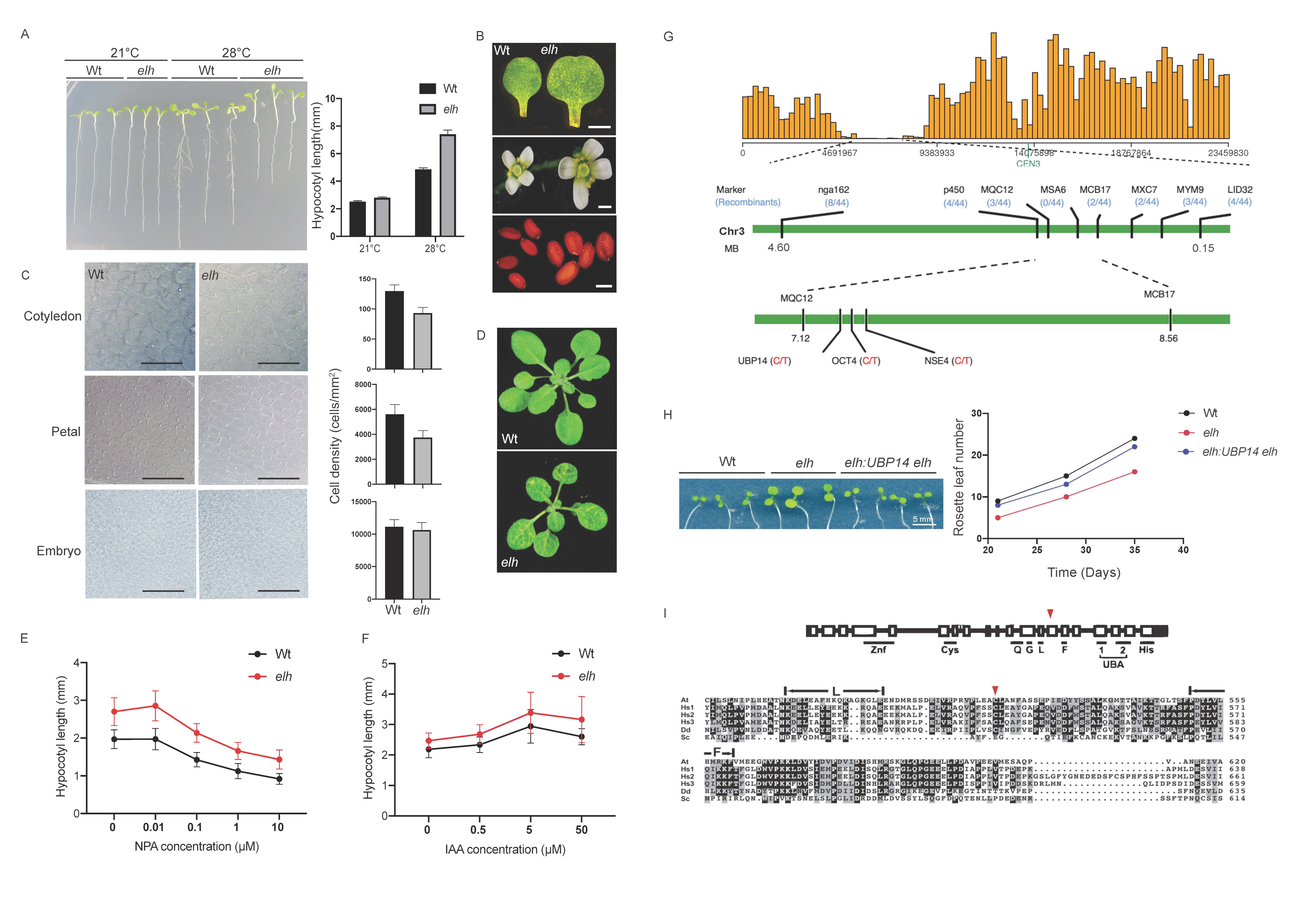
Plant organ size control is an essential process of plant growth and development. The regulation of plant organ size involves a complicated network of genetic, molecular interactions, as well the interplay of environmental factors (Hepworth and Lenhlard, 2014). Two elements of plant organ size development are cell proliferation, resulting in increase in cell numbers, and cell expansion, which increases the overall organ size via larger cell area occupancy. However, our understanding of the fine-tuned regulation of organ size remains incomprehensive. Here, we report a temperature-sensitive hypocotyl elongation EMS-generated mutant, hereby referred to as elongated hypocotyl under high-temperature (elh). The elongated hypocotyl phenotype is prominent when the elh seedlings are grown at high temperature, 28°C, but not under the growth temperature of 21°C (Figure 1A). As hypocotyl elongation is driven primarily by cell elongation processes (Derbyshire et al. 2007), we compared the difference of organs size between in wild type and elh plants. We observed significantly larger organ sizes in elh plants, including cotyledons, petals and seeds when compared to the control (Student’s t-test p < 0.05, Figure 1B). We further inspected the cell size and number of these three organs in wild type and elh plants. In elh, the cell sizes in cotyledons and petals were significantly larger than wild type (Student’s t-test p < 0.05, Figure 1C). We also measured the cell density and organ area of cotyledons, petals and mature dissected embryos. By extrapolating cell numbers based on organ size, we found no differences in total cell numbers in either organ (Student’s t-test p > 0.05) indicating that cell expansion rather than cell proliferation was perturbed in elh. Plastochron, leaf initiation rate, has been previously linked to cell expansion (Wang et al. 2008). We observed reduced leaf number in elh plants when compared to wild type (Student’s t-test p < 0.05, Figure 1D) and a comprehensive analysis showed that elh plants produced leaves at a slower rate than wild type plants (Student’s t-test p < 0.05, Figure 1H), suggesting that perturbing the balance between cell division and cell expansion is linked to the developmental rate at which leaves are produced.
As auxin is a key hormone in regulating hypocotyl elongation and cell expansion (Gray et al. 1998; Chapman et al. 2012) and high auxin levels were shown to increase hypocotyl elongation (Wang et al. 2008; Zhao et al. 2001), we hypothesised that higher auxin levels in elh seedlings may cause the temperature-sensitive hypocotyl elongation phenotype. To test this hypothesis, we introduced the auxin inhibitor 1‐N‐naphthylphthalamic acid (NPA) into both wild-type and elh seedlings (Figure 1E). Higher levels of NPA reduced the hypocotyl length in both wild type and elh seedlings when grown at 28°C. At the concentration of 1 µM NPA, the length of elh hypocotyls were similar to wild type with no NPA (Figure 1 E). When we applied exogeneous auxin, indole-3-acetic acid (IAA) to wild type and elh seedlings (Figure 1F), the hypocotyl length elongation response at 28°C was similar in both genotypes (Figure 1F). Together these results suggest that elh accumulates higher auxin levels leading to a longer hypocotyl when compared to the wild type when grown at 28°C.
To understand the underlying molecular cause of the observed elh phenotypes, we performed bulk segregant analysis combined with Next Generation Mapping (NGM) to identify the causative mutation. Our NGM analysis suggested that the causative mutation in elh resulted in a non-synonymous amino acid substitution from cysteine to tyrosine in the Ubiquitin carboxyl-terminal hydrolase 14 (UBP14; AT3G20630) gene (Figure 1G). UBP14 genetically complemented both the cotyledon size and plastochron phenotypes in transgenic elh plants (Student’s t-test p < 0.05, Figure 1H) demonstrating that UBP14 and ELH are the same gene. UBP14 is essential for early embryo development in Arabidopsis thaliana as null ubp14 mutants are embryo lethal (Doelling et al. 2001). Another UBP14 allele has been shown to regulate organ size via regulating cell cycle kinases which affect both cell numbers and cell expansion (Xu et al. 2016). The UBP14 allele, referred to as da3, has altered mRNA splicing resulting in a premature stop codon and the encoded protein lacks the conserved C-terminal His Box. Here, we characterized a novel UBP14 allele gene that regulates cell organ size and elongation of hypocotyl under high temperature in Arabidopsis. Based on our findings, we postulate that UBP14 regulates organ size via cell expansion through increased levels of auxin that affects the stability of HEAT SHOCK PROTEIN 90 (HSP90) dependent TIF1 co-receptor complexes. It has been shown that high temperatures lead to upregulation of auxin synthesis, followed by the activation of HSP90 to stabilize the TIR1 Auxin/IAA repressor degradation complex (Wang et al. 2016). The elh allele may act to destabilise the HSP90-TIR1 complex, leading to longer hypocotyls (Yamada et al.2009; Wang et al. 2016). Unlike the da3 mutant allele that perturbs both cell expansion and proliferation (Xu et al. 2016), our findings suggest that these two functions can be separated by altering protein function. The elh allele identified here is potentially a useful tool to understand the role of UBP14 in plant development.
Methods
Request a detailed protocolPlant materials and growth
Arabidopsis thaliana (Columbia accession, Col-0) wild type and mutant plants were grown in Phoenix Biosystems chambers under metal halide lights as previously described in David et al. (2017). For plate experiments, seeds were first surface sterilized, plated on ½ MS medium supplemented with 1% sucrose and sealed as previously described in David et al. (2017). All plants were grown under long-day photoperiod conditions of 16 h light and 8 h darkness. For the temperature-sensitive hypocotyl length experiments, seedlings were grown at either 21°C or 28°C.
Auxin inhibitor NPA and exogeneous auxin IAA treatments
For NPA (Sigma Cat no- 33371-100MG) treatment, wild type and elh seeds were germinated on ½ MS media containing various concentration of NPA and along with control plates were transferred at 1 day after germination to 28°C. Seedlings were grown for 7 days and hypocotyl length was measured as described in Gray et al. (1998).
For IAA (Sigma Cat no-I-5148) treatment, seeds were plated on ½ MS media without IAA. Five days post germination, seedlings were transferred to plates containing different concentrations of IAA and allowed to grow for 2 days before hypocotyl length was measured as described in Chapman et al. (2012).
Bulk segregant analysis and next-generation sequencing
To perform a bulk segregant analysis approach, elh was crossed to a polymorphic Landsberg erecta (Ler) parent and in the F2 population, plants that had reduced plastochron were bulked together and sequenced on the Illumina Hiseq platform. The sequencing reads were trimmed using TrimGalore! (https://github.com/FelixKrueger/TrimGalore), trimmed reads were aligned to the TAIR10 reference genome using BWA (Li and Durbin 2010), followed by variant calling using SAMtools (Li et al. 2009). Identification of a SNP deserted region corresponding to ELH was achieved by plotting SNP frequencies across the 5 chromosomes using the Next Generation EMS mutation mapping tool (http://bar.utoronto.ca/ngm/cgi-bin/emap.cgi).
Genetic complementation construct and generation of transgenic plants
For the ELH/UBP14 (AT3G20630) complementation construct, the full-length genomic sequence of ELH/UBP14 including 2 kb upstream promoter sequence was PCR amplified and cloned into the Gateway cloning vector PCR8 TOPO-TA (Invitrogen). The respective insert was sequenced, then cloned into the destination vector pMDC100 via the Gateway Cloning approach (Curtis and Grossniklaus 2003). The construct was transformed into elh plants via Agrobacterium tumefaciens-mediated floral dipping as described by Davis et al. (2009). Transgenic plants were selected on kanamycin containing plates.
Acknowledgments
We thank the ACRF Cancer Genomics (Adelaide, Australia) staff for assistance with the genome sequencing.
References
Funding
This research was supported by an Australian Research Council Future Fellowship (FT130100525) awarded to IRS.
Reviewed By
Stephan WenkelHistory
Received: February 12, 2021Revision received: May 1, 2021
Accepted: May 28, 2021
Published: June 1, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
David, R; Ng, PQ; Smith, LM; Searle, IR (2021). Novel allele elh of the UBP14 gene affects plant organ size via cell expansion in Arabidopsis thaliana.. microPublication Biology. 10.17912/micropub.biology.000401.Download: RIS BibTeX