National Institute of Diabetes and Digestive and Kidney Diseases
Abstract
The C. elegans dauer is an alternative third stage larva induced by dense population and adverse environmental conditions. Genes whose mutants caused dauer formation constitutive (Daf-c) and dauer formation defective (Daf-d) phenotypes were ordered via epistasis into a signaling network, with upstream DAF-7/TGF-beta and DAF-11/receptor guanylyl cyclase defining sensory branches and downstream DAF-2/Insulin receptor and DAF-12/nuclear hormone receptor executing the dauer decision. Mutations in the Scd genes were defined as incompletely penetrant suppressors of the constitutive dauer phenotype conferred by mutation of the DAF-7/TGF-beta signaling axis. SCD-2 was previously shown to be an ortholog of mammalian ALK (Anaplastic Lymphoma Kinase), a receptor tyrosine kinase. Mutations disrupting the HEN-1/Jeb ligand, SOC-1/DOS/GAB adaptor protein and SMA-5/ERK5 atypical MAP Kinase caused Scd phenotypes similar to that of mutant SCD-2. This group regulated expression from a TGF-beta-responsive GFP reporter. Here we find that a strain harboring a mutation in the uncharacterized SCD-4 is mutant for MLK-1, the C. elegans ortholog of mammalian Mixed Lineage Kinase and Drosophila slipper (slpr), a MAP3 kinase. We validated this finding by showing that a previously characterized deletion in MLK-1 caused a Scd phenotype similar to that of mutant SCD-4 and altered expression from the TGF-beta-responsive GFP reporter, suggesting that SCD-4 and MLK-1 are the same protein. Based on shared phenotypes and molecular identities, we hypothesize that MLK-1 functions as a MAP3K in the SCD-2/ALK cascade that signals through SMA-5/ERK5 MAP Kinase to modulate the output of the TGF-beta cascade controlling dauer formation in response to environmental cues.
Description
The C. elegans dauer is an alternative L3 stage larva that forms under harsh environmental conditions, including low food, high temperature, and high concentration of constitutively secreted dauer pheromone. Genetic screens identified genes conferring dauer-constitutive and dauer-defective phenotypes (Daf-c and Daf-d, respectively; Hu, 2007). Double mutant analysis using principles of epistasis and parallelism ordered genes controlling the dauer process into a network (Gottlieb and Ruvkun, 1994; Thomas et al., 1993). Molecular genetic cloning of genes provided identities with similarity to orthologs in Drosophila and mammals. Taken together, these approaches arrived at a model of four main signaling axes controlling entry into dauer: upstream and parallel TGF-beta (DAF-7, mutated to Daf-c) and receptor guanylyl cyclase (DAF-11, mutated to Daf-c) signals reflect parallel processing by sensory neurons, revealed by laser ablation experiments (Birnby et al., 2000; Ren et al., 1996; Schackwitz et al., 1996). Downstream, serial Insulin/IGF-like growth factor receptor (DAF-2, mutated to Daf-c; (Kimura et al., 1997) and nuclear hormone receptor (DAF-12/NHR, mutated to Daf-d; (Antebi et al., 2000) signals control and execute tissue-specific changes in the animal (Fig. 1C, DAF-12 not shown). Mutants for each signaling axis also control diverse developmental and metabolic outputs in addition to the dauer decision. The four-axis model of signaling control of dauer formation neglects potential positive- and negative-feedback loops and is thus likely reductive. Still, these approaches have provided a robust framework for further investigation into the control of the dauer developmental decision by sensory and endocrine signaling modalities.
Genetic screens for mutations that suppress the Daf-c phenotypes conferred by mutations in the DAF-7/TGF-beta signaling cascade identified expected proteins that confer Daf-d phenotypes when mutated (Inoue and Thomas, 2000). Mutants for transcription factors downstream in the TGF-beta signal, DAF-3/co-Smad and DAF-5/Sno-ski (da Graca et al., 2004; Patterson et al., 1997; Tewari et al., 2004), completely suppress Daf-c mutations in the TGF-beta signal. The DAF-16/FoxO transcription factor, downstream of DAF-2/InsR, confers a partial dauer phenotype in double mutant combinations with Daf-c components of TGF-beta signaling (Lin et al., 1997; Ogg et al., 1997). And mutations in DAF-12/NHR, thought to be the most downstream player in the dauer regulatory network, completely suppress mutations in the TGF-beta group that confer a Daf-c phenotype.
Yet this screen also identified mutations – suppressors of constitutive dauer (Scd) – that partially but not completely suppressed the Daf-c phenotype of mutant TGF-beta group genes. These mutations defined three novel genes: scd-1, scd-2 and scd-3 (Inoue and Thomas, 2000). One of these, scd-2, encodes a protein orthologous to Anaplastic Lymphoma Kinase (ALK), a receptor tyrosine kinase that in humans is a proto-oncogene (Reiner et al., 2008). SCD-2/ALK and its putative growth factor ligand HEN-1/Jeb also regulate diverse sensory signals (Ishihara et al., 2002; Kitazono et al., 2017; Shinkai et al., 2011; Wolfe et al., 2019). Mutations in scd-2 at 25˚C strongly but not completely suppressed the DAF-c phenotype of mutant DAF-8/R-Smad, moderately suppressed the Daf-c phenotype of mutant DAF-7/TGF-beta, weakly but consistently suppressed the Daf-c phenotype of mutant DAF-11/rGC, and failed to suppress the Daf-c phenotype of mutant DAF-2/InsR. Through screening to test whether mutations in candidate genes conferred Scd interactions similar to those of mutant SCD-2/ALK, its putative ligand HEN-1/Jeb, adaptor protein SOC-1/DOS/GAB, and ERK5/MAP Kinase SMA-5 were also identified as conferring similar Scd phenotypes. This model was further supported by showing that expression from a transgenic promoter::GFP fusion repressed by DAF-3/Co-Smad (Thatcher et al., 1999) was regulated by mutations in the putative SCD-2/ALK signaling cascade. Taken together, these results suggested that SCD-2/ALK and functionally related genes that confer a similar Scd phenotype when mutated collaborate with the main DAF-7/TGF-beta cascade to co-regulate dauer-regulating genes throughout the animal (Reiner et al., 2008).
A fourth Scd gene, scd-4, was defined by a single allele, sa321, which mapped to chromosome V and thus may have been allelic with scd-2 or soc-1. We previously mapped the phenotype of suppression of daf-7 phenotype of sa321 to the unc-62–dpy-11 interval on chromosome V, excluding the possibility that sa321 was an allele of scd-2, which is located to the right of dpy-11. sa321 also complemented the suppression phenotypes of soc-1(n1789) and scd-2(y386), suggesting that sa321 defined a novel Scd gene, scd-4 (Reiner et al., 2008). sa321 does not alter other phenotypes on plates conferred by mutations in TGF-beta like daf-7(e1372): egg-laying defective (Egl), clumping on the plate (Cpy), and dark intestine (Din; not shown; Thomas et al., 1993).
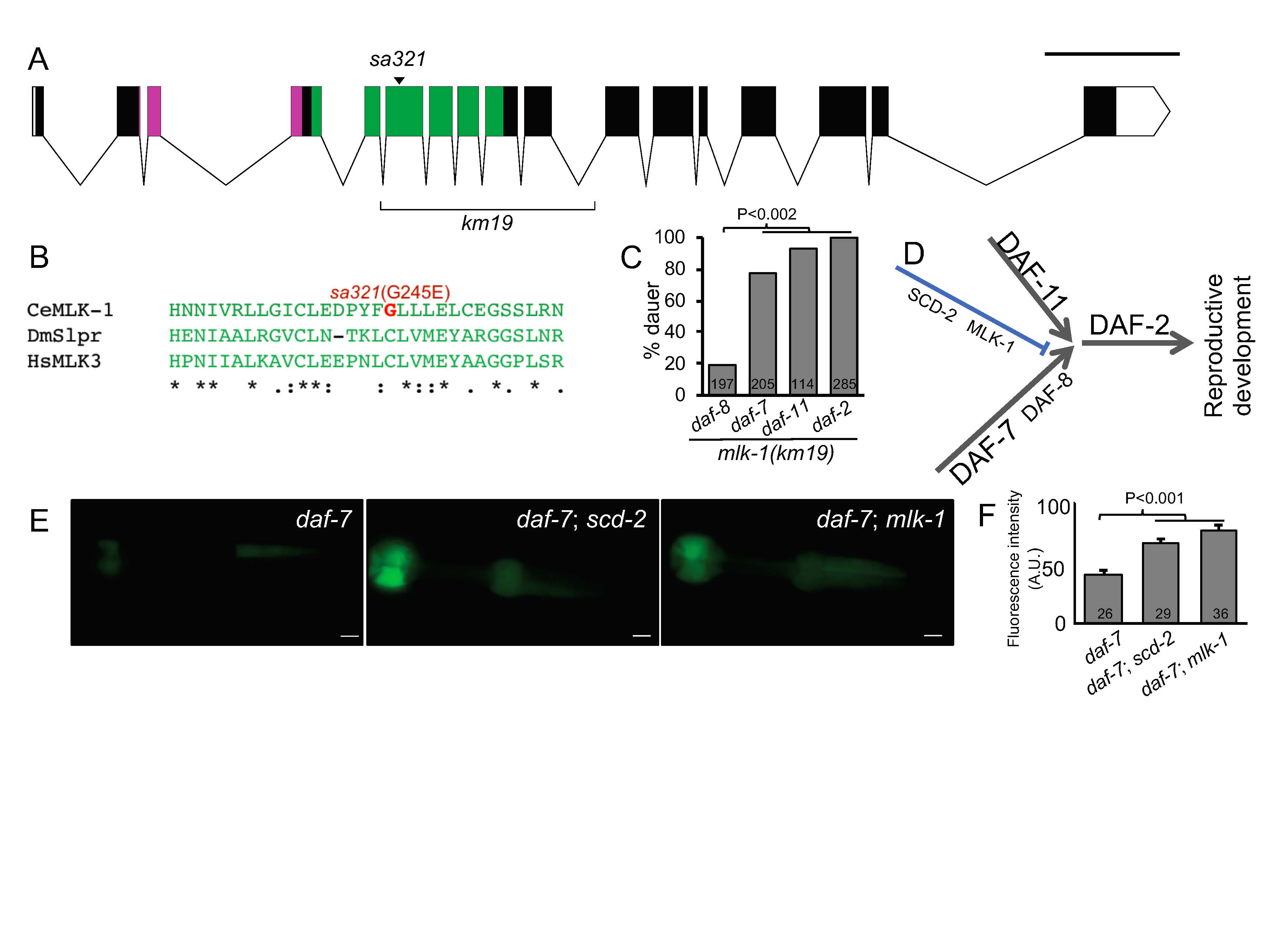
We sequenced the whole genome of the strain JT7478 daf-8(sa234); scd-4(sa321). In the genetic interval of unc-62–dpy-11, we identified non-synonymous mutations in three genes: a G245E mutation in mlk-1, an A189G mutation in ncx-2, and an I5498V mutation in ttn-1. Of these, MLK-1, a MAP3 Kinase most similar to human MLK3 (mixed lineage kinase) and Drosophila slipper (slpr) functions in signal transduction and was a parsimonious candidate for a Scd gene based on our knowledge of SCD-2 as a receptor tyrosine kinase. With Sanger sequencing we confirmed the DNA lesion in mlk-1 of G734A, resulting in the G245E amino acid change in the Ser/Thr kinase domain of MLK-1 (Fig. 1A, B).
To validate that scd-4 is actually mlk-1, we used a deletion allele in mlk-1, km19 (Mizuno et al., 2004). As with mutations in hen-1 and scd-2, sa321 and km19 mutant animals are superficially wild-type when grown on plates. Like sa321 and mutations in scd-2, at 25˚C km19 causes strong but not complete suppression of mutant daf-8, moderate suppression of mutant daf-7, very weak suppression of mutant daf-11, and no suppression of mutant daf-2 (see above; Fig. 1C). Also like scd-2(y386), mlk-1(km19) restored GFP expression from the cuIs2 reporter repressed by daf-7(e1372) (Fig. 1E, quantified in 1F). Furthermore, as observed with certain mutated components of the putative SCD-2/ALK cascade, sa321 and km19 do not alter the Egl, Cpy and Din phenotypes of daf-7(e1372), suggesting that the interaction between MLK-1 and DAF-7/TGF-beta signaling is specific to dauer formation. Thus, we conclude that scd-4(sa321) is an allele of mlk-1. Given their shared mutant phenotypes and identity as signaling molecules, we hypothesize that MLK-1/MAP3K functions downstream of SCD-2/ALK to regulate dauer formation in conjunction with TGF-beta (Fig. 1D), but not other TGF-beta-dependent phenotypes.
Methods
Request a detailed protocolAnimal assays
Animals were grown under standard growth conditions at 15˚C or 25˚ for growth or dauer assays, respectively. Percentage dauer formation was determined from synchronized broods grown at 25˚C laid by parents grown at 15˚C. All assays to be compared were grown in parallel. Response of pharyngeal GFP levels expressed from the cuIs5[Pmyo-2-C-subelement::gfp] to mutational state was as described (Reiner et al., 2008) except for image capture and quantification (see below). These animals were all grown at 15˚C.
Microscopy
Reporter fluorescence was recorded on a Nikon eclipse Ni epifluorescence microscope with DS-Fi2 camera (Nikon) and NIS Elements Advanced research, version 4.40 (Nikon). Images were captured at the same settings and a uniform exposure time of 60 msec with the 40x objective.
Whole genome sequencing
The strain JT7478 daf-8(sa234); scd-4(sa321) was subjected to whole-genome sequencing (50-bp single-end reads, 20-fold genome coverage). Candidate mutations (homozygous, nonsynonymous variants) were identified using a previously described pipeline (Smith and Yun, 2017) and annotated using ANNOVAR (Yang and Wang, 2015).
Reagents
Animal strains used
CB1383 daf-8(e1383) I
DV3650 daf-8(e1383) I; mlk-1(km19) V
CB1372 daf-7(e1272) I
DV3664 daf-7(e1272) I; mlk-1(km19) V
MT4304 daf-11(m47) V
DV3779 mlk-1(km19) daf-11(m47) V
TY1614 unc-62(e644) dpy-11(e224) V
DV3659 unc-62(e644) dpy-11(e224) daf-11(m47) V
CB1370 daf-2(e1370) III
DV3773 daf-2(e1370) III; mlk-1(km19) V
OK43 cuIs5[Pmyo-2-C-subelement::gfp] I
TY3862 cuIs5[Pmyo-2-C-subelement::gfp] I; daf-7(e1372) III
TY3883 cuIs5[Pmyo-2-C-subelement::gfp] I; daf-7(e1372) III; scd-2(y386) V
DV3855 cuIs5[Pmyo-2-C-subelement::gfp] I; daf-7(e1372) III; mlk-1(km19) V
Acknowledgments
We thank Michael Ailion for thawing the strain from the -80 freezer of J. Thomas and sending to the Reiner lab and the lab of Barbara Meyer for sharing strains. We also thank Takao Inoue for the isolation of sa321 and initial mapping to near dpy-11 on chromosome V.
References
Funding
N.R.R. and D.J.R. were funded by NIH grant R01GM121625 to D.J.R.
Reviewed By
AnonymousHistory
Received: May 1, 2021Revision received: May 31, 2021
Accepted: June 2, 2021
Published: June 15, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Rasmussen, NR; Smith, HE; Reiner, DJ (2021). The MLK-1/SCD-4 Mixed Lineage Kinase/MAP3K functions to promote dauer formation upstream of DAF-2/InsR. microPublication Biology. 10.17912/micropub.biology.000405.Download: RIS BibTeX