Abstract
RMD-1,2,3,6 (regulator of microtubule dynamics) is a family of homologous proteins conserved between humans and C. elegans. Human RMD-3/PTPIP51 is a mitochondrial protein that tethers mitochondria to the endoplasmic reticulum. C. elegans RMD-2, 3, and 6 are expressed in sperm. To test whether paternal RMD-2, 3, 6 might redundantly tether paternal mitochondria to maternal ER at fertilization, we generated an rmd-2, rmd-3, rmd-6 triple mutant. Paternal mitochondria derived from control or triple mutant worms were concentrated in a cloud around the paternal DNA at the future posterior end of zygotes during meiosis. No significant difference was detected in the position of paternal mitochondria within the zygote nor in the position of paternal mitochondria relative to paternal DNA within the zygote. There was also no reduction in progeny viability between control and triple mutant worms.
Description
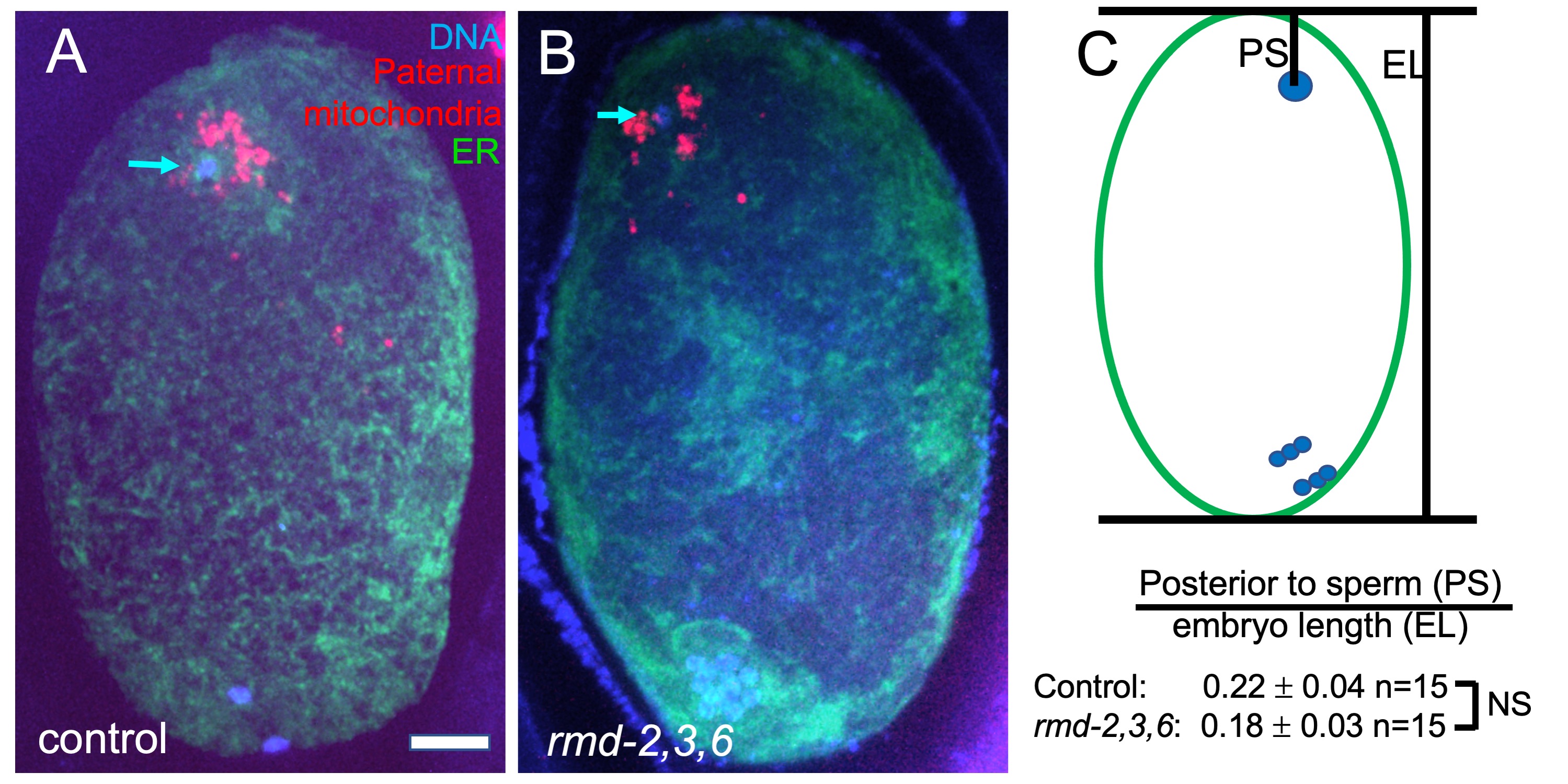
RMD-1 was discovered as a cytoplasmic regulator of microtubule dynamics in C. elegans and several highly homologous proteins were inferred from the C. elegans and human genome sequences (Oishi et al., 2007). One of the human homologs, RMD-3/PTPIP51, has an N-terminal extension relative to other RMDs that targets it to the mitochondrial outer membrane and which binds the endoplasmic reticulum (ER) protein VAPB (De Vos et al., 2012). This interaction mediates tethering of mitochondria to the ER in human cells (Stoica et al., 2014). None of the C. elegans RMDs have sequence homology with the N-terminal extension found on human RMD-3/PTPIP51, however, C. elegans RMD-2 has an N-terminal extension relative to the other RMDs and this extension has potential to form a transmembrane helix as determined by TMpred release 25 (https://embnet.vital-it.ch/software/TMPRED_form.html). RMD-2 is present in sperm whereas RMD-3 and RMD-6 are highly enriched in sperm (Ma et al., 2014). Paternal mitochondria are eventually degraded in the embryo (Sato and Sato, 2011). However, they are maintained, along with other sperm contents, at the opposite end of the zygote from the female meiotic spindle during meiosis (Fig. 1). We hypothesized that RMD-2, 3 and 6 on paternal mitochondria bind to the VAPB homolog, VPR-1, on maternal ER at fertilization to tether the sperm contents at the future posterior end of the zygote.
To test this hypothesis, we mated rmd-2, rmd-3, rmd-6 triple mutant males bearing a transgene labeling mitochondria, mCherry::mev-1 also called mCherry::sdhc-1 (Trewin et al., 2019), to hermaphrodites expressing a fluorescent marker of the ER. Resulting meiotic zygotes were fixed, stained with DAPI, and imaged (Fig. 1A-B). The position of the sperm DNA within the zygote was quantified (Fig. 1C) and no significant difference from control matings was observed. No qualitative difference was observed in distance of paternal mitochondria from the sperm DNA (Fig. 1A, B). Self progeny of triple mutant worms had a hatch rate that was not significantly less than progeny from control worms at 20°C (control KWN724: 94.9% hatching n=6 mothers, 731 progeny; triple deletion FM697: 97.6% hatching n=6 mothers 937 progeny).
Possible explanations are that RMD-1 carries out all the essential functions of this gene family, or that the triple mutant strain has a phenotype that was not assayed.
Methods
Request a detailed protocolrmd-2(du8) was generated by co-injecting KWN724 with two Cas9 RNPs (Upstream Guide Sequence + PAM (rev):
tcagatgtaaagggtgacat cgg
Downstream Guide Sequence + PAM (for):
GCCACCAACTGCCACTGTCG AGG
and the plasmid repair template:
aaatcagaatgataagattaaaaatctccacaatcataaattgaaaagataagatagatctctataaataatgacgtcaactcttgcAATatgtcatct GCCACTGTCGAATAGGCTATCGCCGACTTCCAAGCAGCTCATGAAATCAATCCAGACTGGATTGAAAACACGGTTTACCTCGGAAAAGCTCTTCA
using the dpy-10 co-CRISPR method (Paix et al., 2015). An rmd-2 deletion was identified by PCR screening non-Dpy siblings of Dpy progeny. rmd-2(du8) removes exons 4, 5 and half of exon 6 of rmd-2 isoform b. rmd-6(du9) was generated by co-injecting rmd-2(du8) worms with two Cas9 RNPs (Upstream Guide Sequence + PAM (rev):
ATCGCGGTTCTTGGTTCCGA AGG
Downstream Guide Sequence + PAM (rev):
CGATGTCACACACTTCGGTA AGG
and the ultramer repair template:
ttaatcagtaccgatcaaccaccATGgtaagtttactccgatcttcaaatgtttaagctctttttcagAGTTTTGATGAAATTGACAAGAAATTCGGAAGCAAGTA taggtaactgaTGTGTGACATCGAACCGTATTCCGACGCTGAACAAGAGTTTGCCGATGATGCGAAGCAGATGTTGTCTAAGCTTTAAgttt
using dpy-10 co-CRISPR. PCR screening yielded an rmd-6 deletion in a dpy-10 worm. rmd-6(du9) removes all of exon 3 and half of exons 2 and 4. rmd-3(tm2635) was obtained from Shohei Mitani (National Bioresource Project for the Experimental Animal “Nematode C. elegans”). To make the triple mutant strain, rmd-3(tm2635) males were mated to rmd-2(du8); rmd-6(du9); dpy-10; him-5; mev-1(jbm1 [mev-1::mCherry]) hermaphrodites. F2 self progeny of the F1 heterozygotes were allowed to lay eggs before being subjected to PCR screening for all three deletions. Some genotyping PCRs for tm2635 yielded an apparent wild-type product, however, sequencing of this PCR product revealed it to be from rmd-4, which is annotated as a pseudogene.
Hatch rates were determined by singling L4 hermaphrodites, moving the worm to a fresh plate at 24 hr intervals, then counting eggs and larvae 48 hrs after removing adults from a plate.
Reagents
| strain | genotype | available from |
| KWN724 | mev-1(jbm1 [mev-1::mCherry]) III him-5(e1490) V | McNally lab |
| WH327 | unc-119(ed3) III; ojIs23 [pie-1p::GFP::spcs-1]. | CGC |
| FM697 | rmd-2(du8); rmd-3(tm2635); rmd-6(du9); mev-1(jbm1 [mev-1::mCherry]) III him-5(e1490) V | McNally lab |
Acknowledgments
We thank Shohei Mitani and the National Bioresource Project for the Experimental Animal “Nematode C. elegans” for tm2635, the CGC for WH327, and Keith Nehrke for KWN724.
References
Funding
This work was supported by National Institute of General Medical Sciences grant R35GM136241 and by the U.S. Department of Agriculture/National Institute of Food and Agriculture Hatch project (1009162 to F.J. McNally).
Reviewed By
Paul E. MainsHistory
Received: June 25, 2021Revision received: July 9, 2021
Accepted: July 12, 2021
Published: July 19, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Juanico, IY; Meyer, CM; McCarthy, JE; Gong, T; McNally, FJ (2021). Paternal mitochondria from an rmd-2, rmd-3, rmd-6 triple mutant are properly positioned in the C. elegans zygote. microPublication Biology. 10.17912/micropub.biology.000422.Download: RIS BibTeX