current affiliation: Université Paris-Saclay, CEA, CNRS, Institute for Integrative Biology of the Cell (I2BC), 91198, Gif-sur-Yvette, France
Abstract
Meiotic crossover formation requires the activity of multiple pro-crossover factors, including the MutSγ complex and the cyclin-related protein COSA-1, that become concentrated together at the sites of crossover recombination intermediates. Here we show that a transgene insertion expressing GFP::COSA-1 can suppress the crossover deficit caused by a partial reduction in MutSγ function. Our data, combined with previous findings, support a model in which COSA-1 promotes crossover formation, at least in part, through positive regulation of MutSγ function.
Description
Formation of crossovers (COs) during meiosis depends on the coordinated activities of multiple pro-CO factors, including the MutSγ complex and COSA-1, that become concentrated at the sites of CO-designated recombination intermediates in an interdependent manner (Yokoo et al. 2012; Woglar and Villeneuve 2018). The MutSγ complex is a heterodimer comprising the conserved proteins MSH-5 and MSH-4 (aka HIM-14, encoded by the him-14 gene) (Zalevsky et al. 1999; Kelly et al. 2000). COSA-1 is a member of the cyclin superfamily and is hypothesized to function as part of a cyclin-dependent protein kinase (CDK) complex (Yokoo et al. 2012). The MutSγ complex was suggested to be a potential target for this hypothesized COSA-1 dependent CDK activity, as MSH-5 has multiple potential CDK phosphorylation sites and can be phosphorylated in vitro by a mammalian CDK. These and other data led to a model in which COSA-1-dependent phosphorylation boosts the activity of MutSγ as part of a positive feedback loop that concentrates pro-CO factors at CO sites (Yokoo et al. 2012).
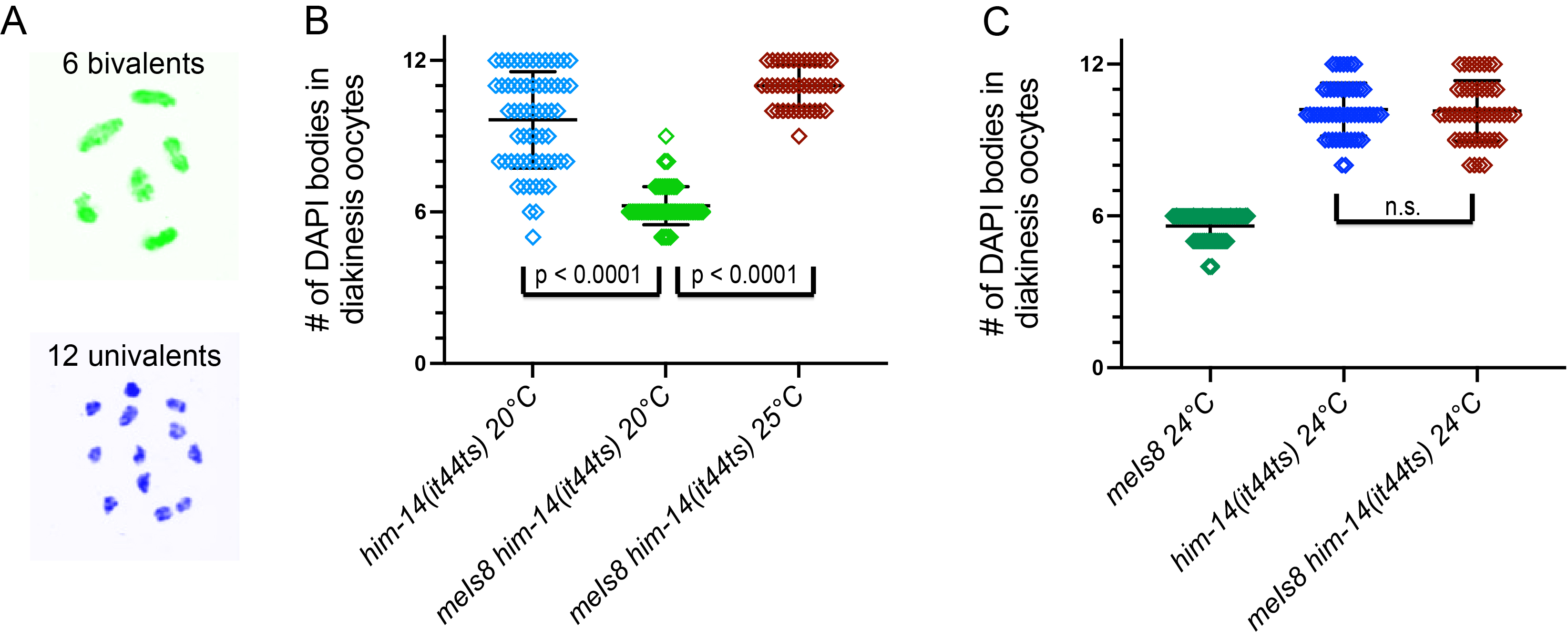
In the course of constructing strains for experiments using the temperature-sensitive missense mutation him-14(it44ts), we obtained additional evidence consistent with a role for COSA-1 as a positive regulator of MutSγ function. Specifically, we found that the presence of meIs8, a transgene insertion expressing a functional GFP::COSA-1 fusion protein (Yokoo et al. 2012), resulted in significant rescue of the CO deficit caused by the him-14(it44ts) mutation at 20°C, a semi-permissive temperature at which MutSγ activity is reduced but not eliminated. We evaluated the success or failure of CO formation by examining DAPI-stained bodies present in oocytes at diakinesis, the last stage of meiotic prophase. Wild-type oocytes contain six DAPI bodies, reflecting six connected homolog pairs (bivalents) harboring CO-based connections (chiasmata), whereas up to 12 DAPI bodies can be resolved in mutants lacking COs, reflecting unconnected individual chromosomes (univalents). In contrast to the high frequency of univalents observed in him-14(it44ts) mutant oocytes at the semi-permissive temperature of 20°C, six bivalents (indicative of successful CO formation for all homolog pairs) were observed in most meIs8 him-14(it44ts) oocytes at 20°C. This finding suggests that an elevated level of COSA-1 protein, due to the presence of the transgene insertion expressing GFP::COSA-1, can increase the likelihood of CO formation in the context of reduced MutSγ activity. However, suppression of the him-14(it44ts) CO defect does not occur at the more restrictive temperatures of 24°C or 25°C where activity of MutSγ is severely impaired. We note that six DAPI bodies are consistently observed at 24°C in diakinesis oocytes of AV818 worms in which meIs8 is the only source of COSA-1 activity (mean = 6.0 ± 0.0, n = 72), indicating that functional GFP::COSA-1 is expressed from meIs8 at 24°C. Together our data suggest that the presence of meIs8 does not bypass the requirement for MutSγ activity to form COs, but instead suppresses him-14(it44ts) by augmenting the residual MutSγ activity present at semi-permissive temperature . Our data are consistent with a model in which COSA-1 promotes CO formation, at least in part, through positive regulation of MutSγ.
Reagents
| Strain | Genotype | Available from |
| KK323 | him-14(it44ts) unc-4(e120)/mnC1 II | AV lab |
| AV893 | meIs8[unc-119(+) pie-1promoter::gfp::cosa-1] him-14(it44ts) unc-4(e120) II | AV lab |
| AV630 | meIs8[unc-119(+) pie-1promoter::gfp::cosa-1] II | CGC |
| AV902 | him-14(it44ts) II | AV lab |
| AV956 | meIs8[unc-119(+) pie-1promoter::gfp::cosa-1] him-14(it44ts) II | AV lab |
| AV818 | meIs8[unc-119(+) pie-1promoter::gfp::cosa-1] cosa-1(tm3298) II | AV lab |
References
Funding
This work was supported by NIH grants R01GM067268 and R35GM126964 to AMV.
Reviewed By
AnonymousHistory
Received: July 15, 2021Revision received: July 31, 2021
Accepted: August 16, 2021
Published: August 24, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Girard, C; Akerib, CC; Villeneuve, AM (2021). Suppression of him-14(it44ts) by a transgene insertion expressing GFP::COSA-1. microPublication Biology. 10.17912/micropub.biology.000430.Download: RIS BibTeX