Abstract
During mating, C. elegans males whose tails have reached the head or tail of their intended mates are able to switch to scanning the other side by performing a turn during which the male’s tail curls ventrally all the while keeping in contact with the hermaphrodite. The ability to execute turns efficiently is dependent upon serotonergic neurons in the posterior ventral nerve cord that stimulate diagonal muscles by activating a serotonin receptor, SER-1. Here we show that turning behavior is abnormal in males lacking a cGMP-dependent protein kinase, EGL-4. egl-4 mutant males are also resistant to ventral tail curling induced by exogenous serotonin.
Description
C. elegans males that have come into close proximity of hermaphrodites initiate copulatory behavior comprising at least five different steps termed response, turning, location of vulva, spicule insertion and sperm transfer (Loer and Kenyon 1993, Liu and Sternberg 1995, Chute and Srinivasan 2014). Mutations specifically affecting different steps have been isolated and characterized (Barr and Sternberg 1999, Hajdu-Cronin et al. 2017, Liu et al. 2017). However, our understanding of the molecular mechanisms acting in the neurons controlling copulation is far from complete. During the response step, males that have sensed the presence of a hermaphrodite move backwards in such a way that the male’s tail fan glides along the surface of the hermaphrodite until the tail reaches the vulva (or head or tail) (Loer and Kenyon 1993, Liu and Sternberg 1995, Sherlekar and Lints 2014). Response behavior is regulated by ciliated neurons in the tail whose dendrites lie in sensory rays within the fan (Liu and Sternberg 1995). If a male reaches the end of the hermaphrodite without having found the vulva, it executes a turn during which the tail bends tightly ventrally so that contact is established between the ventral surface of the fan and the other side of the intended mate (Loer and Kenyon 1993, Liu and Sternberg 1995). The ability to execute turns efficiently is dependent upon serotonergic neurons in the posterior ventral nerve cord (the CP neurons) and on their ability to produce serotonin (Loer and Kenyon 1993, Carnell et al. 2005). Serotonin stimulates the diagonal muscles in the tail to induce curling ventrally by stimulating a serotonin receptor, SER-1 (Loer and Kenyon 1993, Carnell et al. 2005). However, how serotonin affects diagonal muscles and ventral turning is not fully understood.
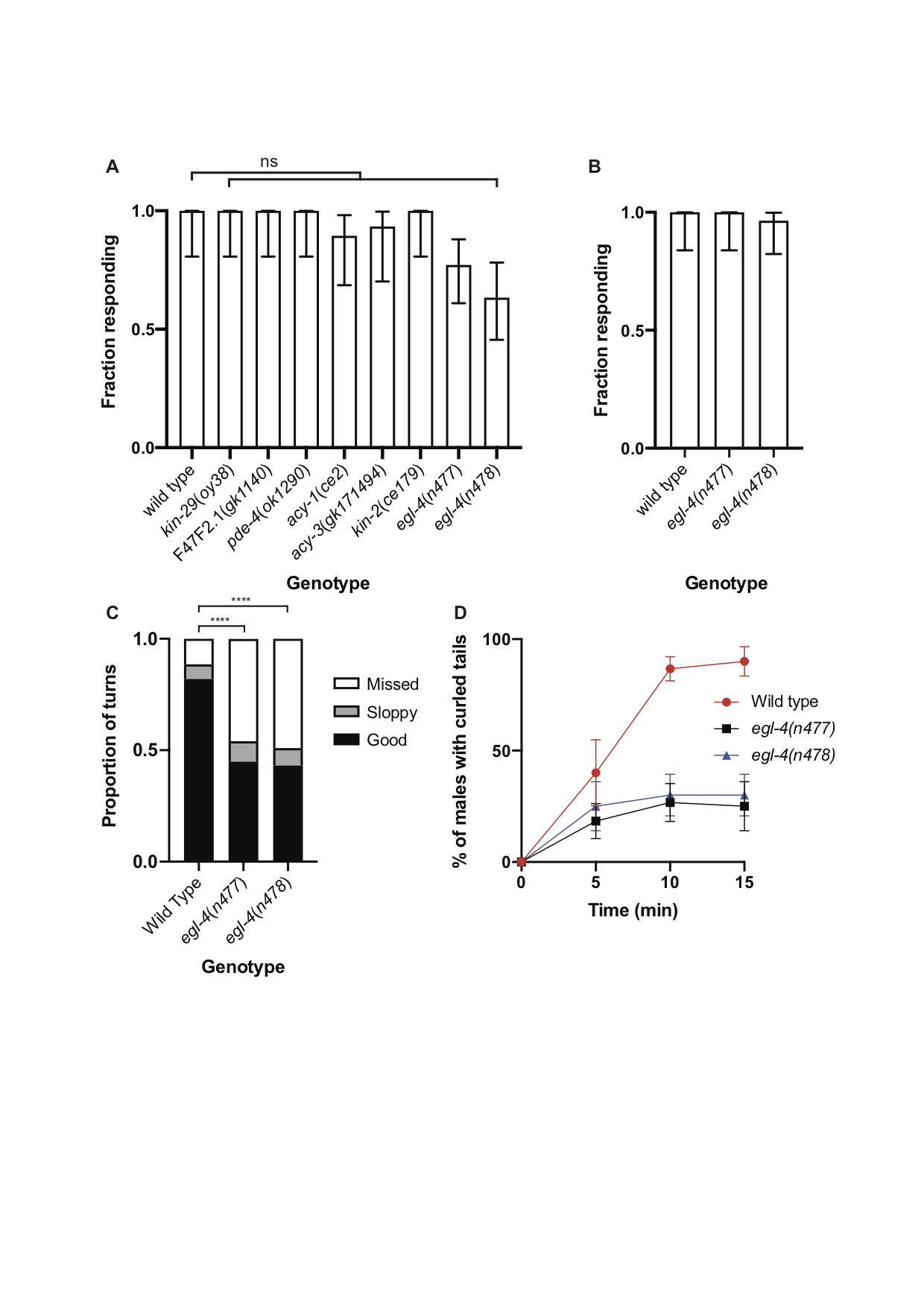
Characterization of the neural networks controlling copulation have indicated that many of the neurons involved have overlapping functions (Liu and Sternberg 1995, Sherlekar and Lints 2014). Furthermore, genetic analysis of copulation has suggested that some of the steps might be regulated by pathways that are genetically redundant. Thus, mutations affecting different steps in copulation might be missed in forward genetic screens. The activities of some sensory neurons in C. elegans and other species are modulated by the cyclic nucleotides cAMP or cGMP (Koelle 2018). An important effector of cAMP is cAMP-dependent protein kinase (protein kinase A), whose regulatory subunit is encoded in C. elegans by kin-2. However, kin-2 mutant males as well those mutant for kin-29, F47F2.1 or pde-4, which all affect cAMP-dependent signaling, were not obviously defective in their response to hermaphrodites (Fig. 1A). An important effector of cGMP in C. elegans is EGL-4, a cGMP-dependent protein kinase (Daniels et al. 2000, Hirose et al. 2003). egl-4 loss-of-function mutant males tended to respond slightly less efficiently than wild-type males in standard response assays (Fig. 1A) but in the presence of a large excess of hermaphrodites almost all egl-4 mutant males were able to respond (Fig. 1B). However, while conducting these assays we noticed that the egl-4 loss-of-function mutant males were defective in their turning behavior and frequently became detached from the hermaphrodite while attempting turns. Quantification of this defect revealed that, compared to those performed by wild-type males, many more of the attempted turns made by egl-4 mutant males were aberrant (Fig. 1C). The turning defect of egl-4 mutant males is similar to that displayed by cat-1 mutant males, which have a reduced ability to synthesize serotonin (Loer and Kenyon 1993). Aberrant turning behavior likely contributes to the reduced ability of egl-4 mutant males to sire progeny in crosses (Daniels et al. 2000).
Application of exogenous serotonin to wild-type males induces tight ventral curling of the tail (Loer and Kenyon 1993). In hermaphrodites, serotonin signaling induces egg-laying and a behavioral state termed dwelling characterized by (among other things) reduced locomotion (Trent et al. 1983, Flavell et al. 2013). Both egg-laying and dwelling behavior are also regulated by egl-4 (Fujiwara et al. 2002, Raizen et al. 2006). Furthermore, extended exposure of wild-type hermaphrodites to serotonin induces paralysis but egl-4 loss-of-function mutant hermaphrodites are partially resistant to paralysis (Olson and Koelle 2019). Consistent with these results, induction of tail curling by exogenous serotonin was less efficient in egl-4 mutant males than in wild type (Fig. 1D). It has previously been shown that whereas SER-1 promotes tail curling, a serotonin-gated chloride channel, MOD-1, has the opposite effect (Carnell et al. 2005). Similarly, mod-5 mutant hermaphrodites are hypersensitive to serotonin-induced paralysis (Olson and Koelle 2019). Thus, models in which EGL-4 either promotes SER-1 activity in diagonal muscles or inhibits MOD-1 activity are consistent with our observations. A further possibility is that EGL-4 acts in a pathway activated by SER-1.
Methods
Request a detailed protocolMale response and turning assays
Males of all genotypes were obtained by RNAi of him-8 and klp-16 (Timmons et al. 2014). Turning behaviour, and the response of males to hermaphrodites were assessed by procedures described previously (Loer and Kenyon 1993, Liu and Sternberg 1995). In both cases, wild-type hermaphrodites were used in the assays. For the turning assays, more than 60 turns/attempted turns were observed for each genotype. More than 20 different males were observed for each genotype. The analysis of tail curling in response to exogenous serotonin was carried out as previously described (Loer and Kenyon 1993). Males were placed in the wells of a microtiter plate and serotonin hydrochloride (obtained from Sigma-Aldrich) was added to give a final concentration of 20 mM.
Reagents
Strain list.
| Strain | Genotype | Available from |
| N2 | Caenorhabditis elegans | CGC |
| MT1072 | egl-4(n477) | CGC |
| MT1073 | egl-4(n478) | CGC |
| KG518 | acy-1(ce2) | CGC |
| VC2531 | F47F2.1(gk1140) | CGC |
| KG532 | kin-2(ce179) | CGC |
| PY1479 | kin-29(oy38) | CGC |
| RB1231 | pde-4(ok1290) | CGC |
| VC20501a | acy-3(gk171494) | CGC |
aThis strain is from the million-mutation project and contains mutations in other genes.
References
Funding
The work was supported by a grant from Insamlingsstiftelsen
Reviewed By
AnonymousHistory
Received: July 7, 2021Revision received: August 11, 2021
Accepted: August 11, 2021
Published: August 18, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Rahmani, S; Tuck, S (2021). EGL-4 promotes turning behavior of C. elegans males during mating. microPublication Biology. 10.17912/micropub.biology.000433.Download: RIS BibTeX