Abstract
The first generation of C. elegans GAL4 drivers for bipartite expression function less well than C. elegans tet ON/OFF, QF and LexA drivers. The main difference between the GAL4 drivers and the others is the absence of a flexible linker between the DNA binding and activation domain in the GAL4 construct. Addition of a linker to a GAL4-QF construct increased driver potency, while adding linkers to a GAL4-VP64 driver was much less effective. Extending the linker region of the tetR-L-QF driver also increased activity of that driver. The new GAL4 driver makes GAL4/UAS bipartite system activity comparable to the other worm bipartite expression systems.
Description
Several bipartite systems have been described for use with C. elegans (Wei et al., 2012; Wang et al., 2017; Wang et al., 2018; Mao et al., 2019; Nonet, 2020). However, little manipulation of the drivers or reporters has been performed to optimize them for C. elegans. Mao. et. al. 2019 demonstrated that the QF activation domain is a more potent activator than either VP64 or the hybrid VP64-p65-Rta tripartite activatorVPR. Here, I describe modifications of linker region between the activation domains of both GAL4SK and TetR drivers that increase the activity of these reporters. Although these studies are not comprehensive, I opted to describe them herein because the modification of the GAL4SK driver increases the activity of this driver substantially such that it is now on par with the LexA, TetR and QF2 drivers.
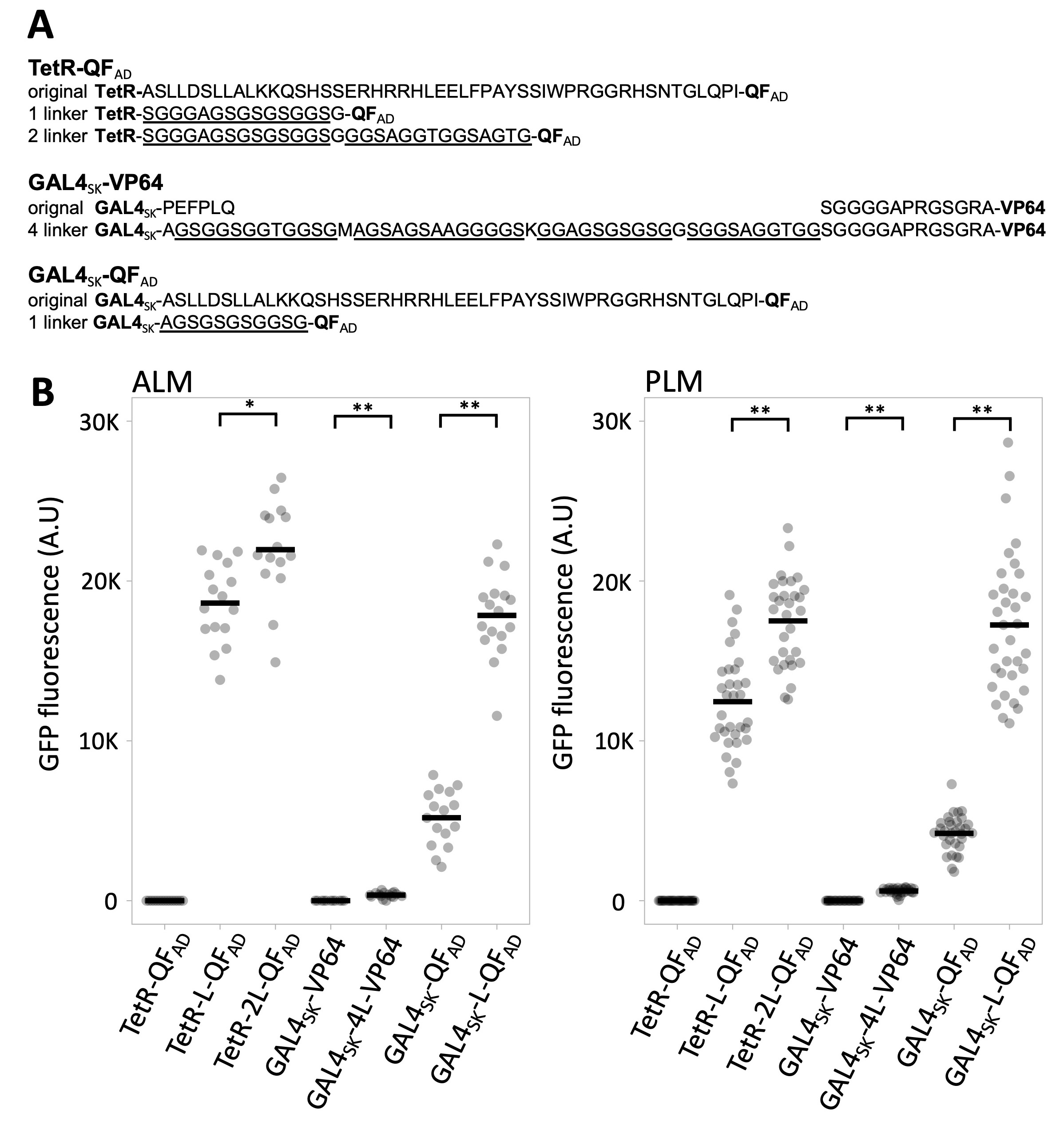
I used an efficient RMCE protocol (Nonet, 2020) to create the transgenic animals. Modified versions of a mec-4 promoter GAL4SK-QFAD, GAL4SK-VP64 and TetR-QFAD constructs were created in an RMCE integration vector using a Golden Gate cloning approach, then integrated on Chr IV using a standard injection protocol. After outcrossing to the appropriate reporter, the expression level of GFP at steady state in PLM and ALM soma of L4 animals was quantified (Figure 1).
Previously, I described drivers consisting of C. elegans codon optimized synthetic GAL4SK, TetR and LexA DNA binding domains fused to the QF activation domain based on the observations of Mao. et al. 2019. Although the GAL4SK construct was modestly active, the LexA and TetR construct were incapable of activating the reporter (supplemental methods of Nonet, 2020). Replacement of a 49 amino acid portion of central domain of QF, which separates the TetR and LexA DNA binding domains and the QF activation domain in the original constructs, with a 12 amino acid flexible linker converted both into much stronger drivers than the GAL4SK-QFAD driver (Nonet, 2020). Here I show that insertion of a similar linker also greatly increases the potency of the GAL4SK-QFAD driver. I also extended the linker of the TetR-L-QFAD and this further improved activity of this driver. In Nonet, 2020, I also tested the functionality of a GAL4SK-VP64 construct (Wang et al. 2017) in single copy and found it was incapable of expressing the GFP reporter. To test if the failure of GAL4SK-VP64 was also the result of insufficient domain separation, I inserted a 40 amino acid flexible linker in between GAL4 and VP64. Although the linker containing driver activates transcription, it does so extremely poorly in comparison to the GAL4SK-QFAD drivers. I speculate this is likely due to the loss of the MED25 subunit of the mediator complex in the nematode lineage (Grants et al., 2015). VP64 (a 4X VP16) is known to activate transcription in part through interaction with MED25 (Mittler et al., 2003) and the loss of this interaction could account for the observed weak activation properties of VP64 and related activators in worms (Mao et al. 2019 and herein).
In addition to the modification of the linker domain of the transgenes I characterize herein, some of the transgenes also differ in other ways that could theoretically impact my conclusions. First, some driver transgenes contain a tbb-2 3′ UTR and others use an act-4 3′ UTR. I consider it very unlikely these differences impact GFP expression for two reasons. First, my lab has previously demonstrated that mec-4promoter driven GFP-C1 transgenes employing the tbb-2 3′ UTR and the act-4 3′ UTR express at very similar levels in ALM and PLM (Dour and Nonet, 2021). More importantly, I previously demonstrated that the activity of the both a GAL4-QFAD driver and a LexA-L-QFAD driver are insensitive to dosage of the driver (Figure 5 of Nonet, 2020). Specifically, GFP levels observed in GAL4SK/+; UAS::GFP ~= GALSK; UAS::GFP. Thus, the level of expression of the driver is unlikely to be determining the GFP signal level. Rather, I speculate that GAL4SK is saturating the UAS binding sites in all transgenes and that the inherent activation properties of the driver determines the expression level.
Second, all drivers were integrated at jsTi1493 except for the inactive linkerless TetR-QFAD driver. I present data on integration of NMp3730 (mec-4p tetR-QFAD tbb-2 3′ UTR) at jsTi1490 (Nonet, 2020). I also previously created insertions of the same plasmid at jsTi1493 IV and jsTi1492 II (jsSi1531 [mec-4p tetR-QFAD tbb-2 3′] IV and jsSi1534 [mec-4p tetR-QFAD tbb-2 3′] II; Table S1 and Table S5 in Nonet, 2020). The GFP expression was undetectable in jsSi1531/+; jsSi1519 [7X tetO ∆pes-10p GFP]/+ and jsSi1534/+;jsSi1519/+ double heterozygote animals. However, only the jsTi1490 transgene was homozygosed with the reporter. Thus, I quantified that insertion. However, this difference is highly unlikely to impact my findings as the other two insertions are qualitatively completely inactive.
The improvements to GAL4SK-QFAD by insertion of a flexible linker is an important addition to the C. elegans bipartite expression toolkit since the GAL4 system is so extensively developed in Drosophila. Using multi-copy lines and a VP64 activator, Wang et al. (2018) have already shown that the split GAL4SK system is functional in worms. Incorporation of a similar flexible linker and a QF activation domain into those tools should permit development of a robust single copy split-GAL4SK system. Other GAL4 system tools previously developed for Drosophila such as GAL80ts and GAL4-PR tools (Caygill and Brand, 2016) which provide temporal control in addition to spatial control could also easily be incorporated into the worm toolbox.
In addition, further manipulation of the size and properties of the linker domain separating the DNA binding domain and activation domains could yield drivers with even stronger activation proper which would likely also be applicable to the LexA, QF and Tet ON/OFF driver/reporter systems.
Methods
Request a detailed protocolMethods
C. elegans was maintained on NGM agar plates spotted with OP50 at 22.5°C or at 25°C during the RMCE protocol.
RMCE transgenesis
Inserts were cloned into pLF3FShC (Addgene # 153083; Nonet, 2020) and injected at ~50 ng/µl into jsTi1493 young adults. Integrants were identified and isolated as described in detail in Nonet (2020). The GAL4SK drivers were crossed to jsSi1518 [11X UAS ∆pes-10 GFP-C1] and the TetR strains were crossed to jsSi1543 [7X tetO ∆mec-7p GFP-C1].
Microscopy
For quantification of GFP signals, homozygous L4 hermaphrodite animals were mounted on 2% agar pads in a 2 µl drop of 1mM levamisole in phosphate buffered saline and imaged on an Olympus (Center Valley, PA) BX-60 microscope equipped with a Qimaging (Surrey, BC Canada) Retiga EXi monochrome CCD camera, a Lumencor AURA LED light source, Semrock (Rochester, NY) GFP-3035B and mCherry-A-000 filter sets, and a Tofra (Palo Alto, CA) focus drive, run using micro-manager 2.0ß software (Edelstein et al., 2014) using a 40X air lens at 20% LED power with 50 ms exposures. PLM soma and ALM soma signals were quantified using the FIJI version of ImageJ software (Schindelin et al., 2012) as described in Nonet (2020).
Plasmid constructions
Integration vectors were assembled using Golden Gate (GG) reactions as described in Nonet (2020). Other plasmids were constructed using standard cloning techniques.
The following were used:
NMp3055 DR274 U6 (Table S3 and Supplemental methods of Nonet, 2020)
NMp3401 DR274 CT linker
NMp3055 was digested with EcoRI and HindIII and the double stranded (ds) oligonucleotide NMo5948/49 was ligated into the vector.
NMp3403 DR274 CT-FP linker
NMp3055 was digested with EcoRI and HindIII and the ds oligonucleotide NMo5952/53 was ligated into the vector.
NMp3498 DR274 FP linker
NMp3403 was modified by DpnI-mediated mutagenesis using oligonucleotides NMo6120/21.
NMp3610 syn Gal4SK DB (Table S3 and Supplemental methods of Nonet, 2020)
NMp3617 pSAP mec-4p GALSK-VP64 (Table S3 and Supplemental methods of Nonet, 2020)
NMp3643 pLF3FShC Available at Addgene. (Table S3 and Supplemental methods of Nonet, 2020)
NMp3735 DR274 5’arm- CT mec-4p (Table S3 and Supplemental methods of Nonet, 2020)
NMp3736 DR274 TGG GGT mec-4p (Table S3 and Supplemental methods of Nonet, 2020)
NMp3751 DR274 AAG GTA act-4 3’UTR (Table S3 and Supplemental methods of Nonet, 2020)
NMp3777 DR274 3’arm tbb-2 UTR (Table S3 and Supplemental methods of Nonet, 2020)
NMp3808 DR274 CT-NT linker-QFAD (Table S3 and Supplemental methods of Nonet, 2020)
NMp3821 DR274 FP tetR(iS)-L-QFAD (called DR274 FP tetR(iS)-L-QF in Table S3 and Supplemental methods of Nonet, 2020)
NMp3876 pLF3FShC mec-4p GAL4SK-L-QFAD
NMp3736, NMp3610, NMp3808 and NMp3777 were co assembled into NMp3643 using a SapI GG reaction.
NMp4045 DR274 FP tetR-LL-QFAD
NMp3821 was amplified with oligonucleotides NMo6867 and NMo7056, DpnI digested, purified, kinased, and religated.
NMp4048 pLF3FShC mec-4p tetR-LL-QFAD act-4
NMp3735, NMp4045, and NMp3751 were co assembled into NMp3643 using a SapI GG reaction.
NMp4049 pLF3FShC mec-4p GAL4SK-L4-VP64 tbb-2 3’UTR
NMp3736, NMp3610, NMp3401, NMp3498, NMp3777, the ds oligonucleotide NMo6866/67, and VP64 as a PCR product amplified from NMp3617 using oligonucleotides NMo6404/7057 were co assembled into NMp3643 using a SapI GG reaction.
Oligonucleotides
| NMo number | Sequence |
| 5948 | AATTGCTCTTCgGCGGGCAGCGGTGGCAGTGGAGGTACCGGCGGAAGCGGTATGcGAAG |
| 5949 | AGCTCTTCgCATACCGCTTCCGCCGGTACCTCCACTGCCACCGCTGCCCGCcGAAGAGC |
| 5952 | AATTGCTCTTCgGGTGGCAGCGCTGGAGGTACCGGCGGTAGTGCCGGAGGCACGcGAAG |
| 5953 | AGCTCTTCgCGTGCCTCCGGCACTACCGCCGGTACCTCCAGCGCTGCCACCcGAAGAGC |
| 6120 | GCTCTTCAATGGCCGGCTCCGCCGGCTCT |
| 6121 | CGGAGCCGGCCATTGAAGAGCAATTCAAAAATCATACC |
| 6404 | CAAGCTCTTCGCGTTTAGTTAATCAGCATGTCCAGG |
| 6866 | AAGGGAGGAGCGGGTTCTGGATCTGGATCTGGAGGTTCC |
| 6867 | ACCGGAACCTCCAGATCCAGATCCAGAACCCGCTCCTCC |
| 7056 | GGTGGCAGCGCTGGAGGTACCGGCGGTAGTGCCGGAACGggtcgtcaacttga |
| 7057 | AATGCTCTTCaGGTGGCAGCGCTGGAGGTACCGGCGGTTCTGGTGGCGGAGGG |
Transgenes
| Name | Description | Full Designation | Comments |
| jsTi1493 IV | Chr IV landing site | jsTi1493 [mosL loxP mex-5p FLP sl2 mNeonGreen rpl-28p FRT GFP-HIS-58 FRT3 mosR] IV | Nonet, 2020 |
| jsSi1515 IV | mec-4p::GAL4SK-QFAD | jsTi1493 jsSi1515 [mosL loxP mec-4p GAL4SK-QFAD tbb-2 3′ FRT3 mosR] IV | Nonet, 2020 |
| jsSi1516 IV | mec-4p::tetR-QFAD | jsTi1490 jsSi1516 [mosL loxP mec-4p tetR-QFAD tbb-2 3′ FRT3 mosR] IV | Nonet, 2020 |
| jsSi1518 I | 11X UAS::GFP | jsTi1453 jsSi1518 [mosL loxP 11X UAS ∆pes-10p GFP-C1 tbb-2 3′ FRT3 mosR] I | Nonet, 2020 |
| jsSi1519 I | 7X tetO ∆pes-10::GFP | jsTi1453 jsSi1519 [mosL loxP 7X tetO ∆pes-10p GFP-C1 tbb-2 3′ FRT3 mosR] I | Nonet, 2020 |
| jsSi1525 IV | mec-4p::GAL4SK-VP64 | jsTi1493 jsSi1525 [mosL loxP mec-4p GAL4SK-VP64 tbb-2 ‘3 FRT3 mosR] IV | Nonet, 2020 |
| jsSi1543 I | 7X tetO ∆mec-7p::GFP | jsTi1453 jsSi1543 [mosL loxP tetO 7X ∆mec-7p GFP-C1 tbb-2 3′ FRT3 mosR] I | Nonet, 2020 |
| jsSi1560 IV | mec-4p::tetR-L-QFAD | jsTi1493 jsSi1560 [mosL loxP mec-4p tetR-L-QFAD act-4 3′ FRT3 mosR] IV | Nonet, 2020 |
| jsSi1588 IV | mec-4p::GAL4SK-L-QFAD | jsTi1493 jsSi1588 [mosL loxP mec-4p GAL4SK-L-QFAD act-4 3′ FRT3 mosR] IV | RMCE insertion of NMp3876 into jsTi1493 |
| jsSi1661 IV | mec-4p::tetR-L-QFAD | jsTi1493 jsSi1661 [mosL loxP mec-4p tetR-LL-QFAD act-4 3′ FRT3 mosR] IV | RMCE insertion of NMp4048 into jsTi1493 |
| jsSi1664 IV | mec-4p::GAL4SK-L4-VP64 | jsTi1493 jsSi1664 [mosL loxP mec-4p GAL4SK-L4-VP64 tbb-2 3’ FRT3 mosR] IV | RMCE insertion of NMp4049 into jsTi1493 |
Worm Strains
| NM strain | Genotype | Source |
| 5179 | jsTi1493 [mosL loxP mex-5p FLP sl2 mNeonGreen rpl-28p FRT GFP-HIS-58 FRT3 mosR] IV | Nonet, 2020; CGC |
| 5213 | jsTi1453 jsSi1518 [mosL loxP 11X UAS ∆pes-10p GFP-C1 tbb-2 3′ FRT3 mosR] I; him-8(e1489) IV | Nonet, 2020 |
| 5214 | jsTi1453 jsSi1519 [mosL loxP 7X tetO ∆pes-10p GFP-C1 tbb-2 3′ FRT3 mosR] I; him-8(e1489) IV | Nonet, 2020 |
| 5225 | jsTi1453 jsSi1519 [mosL loxP tetO 7X ∆pes-10p GFP-C1 tbb-2 3′ FRT3 mosR] I; jsTi1490 jsSi1516 [mosL loxP mec-4p tetR-QFAD tbb-2 3′ FRT3 mosR] IV | This work |
| 5233 | jsTi1453 jsSi1518 loxP UAS 11X ∆pes-10p GFP-C1 tbb-2 3′ FRT3 mosR] I; jsTi1493 jsSi1515 [mosL loxP mec-4p GAL4SK-QFAD tbb-2 3’ FRT3 mosR] IV | Nonet, 2020; CGC |
| 5264 | jsTi1453 jsSi1543 [mosL loxP tetO 7X ∆mec-7p GFP-C1 tbb-2 3′ FRT3 mosR] I; him-8(e1489) IV | Nonet, 2020 |
| 5295 | jsTi1493 jsSi1560 [mosL loxP mec-4p tetR-L-QFAD act-4 ‘3 FRT3 mosR] IV | Nonet, 2020 |
| 5301 | jsTi1453 jsSi1518 [mosL loxP UAS 11X ∆pes-10p GFP-C1 tbb-2 3′ FRT3 mosR] I; jsTi1493 jsSi1525 [mosL loxP mec-4p GAL4SK-VP64 tbb-2 3’ FRT3 mosR] IV | Nonet, 2020 |
| 5353 | jsTi1493 jsSi1588 [mosL loxP mec-4p GAL4SK-L-QFAD act-4 3′ FRT3 MosR] IV | This work |
| 5362 | jsTi1453 jsSi1518 [mosL loxP UAS 11X ∆pes-10 GFP-C1 tbb-2 3′ FRT3 mosR] I; jsTi1493 jsSi1588 [mosL loxP mec-4p GAL4SK-L-QFAD act-4 3′ FRT3 mosR] IV | This work |
| 5467 | jsTi1453 jsSi1543 [mosL loxP tetO 7X ∆mec-7p GFP-C1 tbb-2 3′ FRT3 mosR] I; jsTi1493 jsSi1560 [mosL loxP mec-4p tetR-L-QFAD act-4 3’ FRT3 mosR] IV | This work |
| 5468 | jsTi1453 jsSi1543 [mosL loxP tetO 7X ∆mec-7p GFP-C1 tbb-2 3′ FRT3 mosR] I; jsTi1493 jsSi1661 [mosL loxP mec-4p tetR-LL-QFAD act-4 3′ FRT3 mosR 3′ ] IV | This work |
| 5470 | jsTi1453 jsSi1518 [mosL loxP UAS 11X ∆pes-10 GFP-C1 tbb-2 3′ FRT3 mosR] I; jsTi1493 jsSi1664 [mosL loxP mec-4p GAL4SK-L4–QFAD tbb-2 3’ FRT3 mosR] IV | This work |
Reagents
Plasmids and worm strains are available by request from MLN and will be submitted to Addgene and the Caenorhabditis Genetics Center if demand levels warrant it.
References
Funding
WUMS department of neuroscience funds.
Reviewed By
Han Wang and AnonymousHistory
Received: July 3, 2021Revision received: July 27, 2021
Accepted: September 7, 2021
Published: September 14, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Nonet, M (2021). Improved GAL4 and Tet OFF drivers for C. elegans bipartite expression. microPublication Biology. 10.17912/micropub.biology.000438.Download: RIS BibTeX