Molecular Mycology laboratory, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore, Karnataka
Abstract
CCT (Chaperonin containing TCP-1) is a constitutively expressed eukaryotic chaperonin complex involved in the proper folding of proteins like actin and tubulin. Temperature sensitive mutants of CCT complex have been employed in various genetic screens, acting as models to study human CCT, the defects of which are implicated in disease conditions like neurodegeneration. Mutants of CCT complex are sensitive to cell wall stress agents. In this study, we have tested the effects of sorbitol and protein kinase C overexpression on the temperature sensitivity of cct mutants. We report that both the factors alleviated temperature sensitivity of cct mutants, indicating the possible role of CCT in maintaining cell wall integrity in S. cerevisiae.
Description
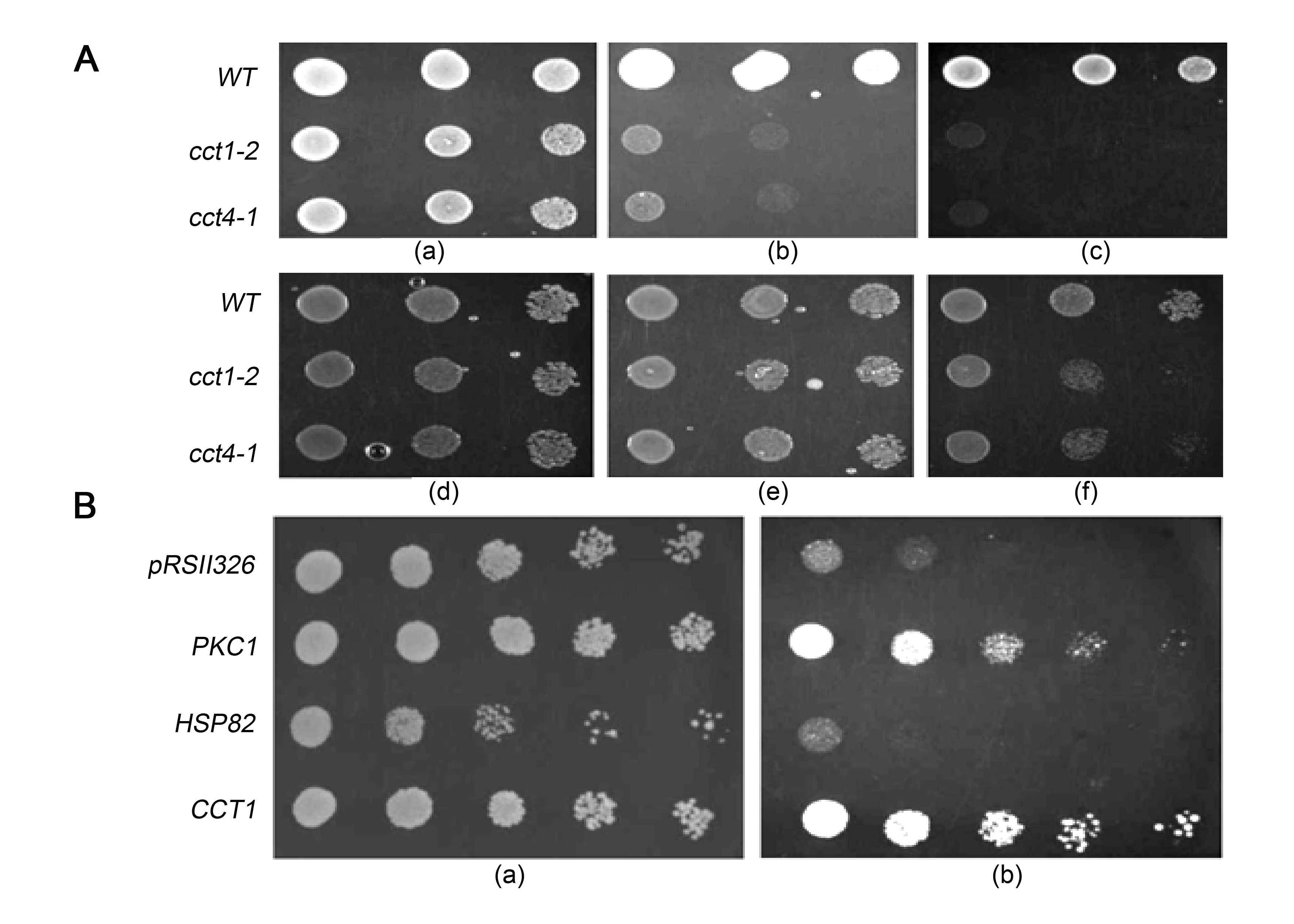
TRiC (TCP-1 Ring Complex) or CCT (Chaperonin Containing TCP-1) is a multi-subunit, double-ringed, ATP-driven cytosolic eukaryotic chaperonin complex that is constitutively expressed and facilitates the folding of several proteins like actin and tubulin (Frydman et al. 1992; Llorca et al. 1999; Berger et al. 2018). Its role in many cellular processes like differentiation, viral replication, and heart contractility were elucidated recently (Melkani et al. 2017; Knowlton et al. 2018; Ohhara et al. 2019). Its functional defects are also associated with neurodegenerative diseases and cancer (Tam et al. 2006; Boudiaf-Benmammar et al. 2013). Temperature sensitive mutants of Saccharomyces cerevisiae CCT have been used in different studies, especially those involving genetic screens to identify CCT-interacting proteins (Kabir and Sherman 2008). The CCT1–CCT8 genes encode eight different subunits of the complex; the mutant allele cct1-2 has G423D replacement in the conserved ATP binding/hydrolysis domain in the subunit Cct1p. cct4-1 (G345D), the mutation in subunit Cct4p, affects ATP binding, intra and inter-ring co-operativity (Shimon et al. 2008). cct1-2 and cct4-1 grew well at 30°C (permissive temperature), showed retarded growth at 35°C (semi-permissive temperature) and failed to grow at 37°C (restrictive temperature).
Their sensitivity to cell wall antagonists like SDS and congo red are suggestive of cell wall defects in cct mutants (Narayanan et al. 2016). Are the temperature sensitivity and cell wall defects linked in cct mutants? To answer this, we studied the effects of two different suppressors of cell wall defects on the temperature sensitivity of cct mutants.
Osmotic stabilisers like sorbitol are known to rescue cell-wall integrity mutants by preventing cell lysis (Ribas et al. 1991). We observed that the growth of cct1-2 and cct4-1 mutants was restored in presence of 1M sorbitol both at the semi-permissive and restrictive temperatures (Fig. 1A). Mutants in MAPK-activation pathway undergo cell lysis at elevated temperatures, due to the unrepaired cell wall defects (Kamada et al. 1995). The osmoremedial nature of temperature sensitivity in the cct mutants prompted us to study the role of cell wall biogenesis in temperature sensitivity. Towards this end, we overexpressed the PKC1 gene in cct1-2. The PKC1 gene in S. cerevisiae encodes protein kinase C, homolog of human protein kinase C, an important enzyme in the maintenance of cell wall integrity (Levin 2005). Overexpression of PKC1 resulted in the rescue of the temperature-sensitive phenotype of cct mutants at 35°C and 37°C (Fig. 1B).
Wild type copy of the CCT1 gene was also overexpressed that served as the positive control; the overexpression enabled growth at 35°C and 37°C, confirming that the defects observed in the mutant were indeed due to compromised CCT function. HSP82, a yeast HSP90 homolog, was overexpressed in cct1-2 to check if more copies of another chaperone can reverse the defects in the mutant. But, unlike PKC1, HSP82 on overexpression failed to make an impact on the temperature sensitivity of cct mutants. Similar observations were made for HSP40 and HSP90– overexpression of PKC1 was found to ameliorate the temperature sensitivity in these heat shock protein mutants (Wright et al. 2007). Our results suggest the role of CCT, a constitutively expressed cytosolic chaperonin, in maintaining cell wall integrity in S. cerevisiae.
Methods
Request a detailed protocolYeast extract (1%)- Peptone (2%)- Dextrose (2%) (YPD) medium was used to grow the yeast strains. The cct1-2 and cct4-1 strains were grown at different temperatures in media with and without sorbitol to study the effect of osmotic stabilizers. DUY326 was transformed with pFR22 (YEpU-PKC1) and pCMS154-HSP82 for the overexpression of PKC1 and HSP82, respectively (Wright et al. 2007) using the lithium acetate/PEG method. The transformants were selected on uracil-omission medium. The plates were incubated at 30 °C for two days and the transformants were subcultured in uracil-omission medium. Approximately equal number of cells of the transformants were serially diluted, spotted on YPD and incubated at different temperatures to study the phenotype.
Reagents
| Plasmid | Description | Reference/Source |
| pRSII326 | 2- micron based multicopy plasmid with URA3 | Chee et al. 2012 |
| YEpU-PKC1 | PKC1 cloned in YEp352 | Wright et al. 2007 |
| pCMS154-HSP82 | HSP82 cloned in pRS426 | Wright et al. 2007 |
| pAB2477 | CCT1 URA3 CEN/ARS |
| Strain | Genotype | Figure | Source |
| B-8728 | MATa ura3-52 trp1-Δ63 leu2-Δ1 GAL2 | Figure 1A | Lin et al. 1997 |
| DUY326 | MATa ura3-52 lys2-801 ade2-201 cct1-1 leu2-3,-112 | Figure 1A | T C Huffaker |
| DDY229 | MATa cct4-1 leu2-3,112 lys2-801 ura3-52 | Figure 1A | D G Drubin |
| D094 | DUY326 transformed with pAB2477 | Figure 1B | This study |
| D352 | DUY326 transformed with pRS11326 | Figure 1B | This study |
| D353 | DUY326 transformed with YEpU-PKC1 | Figure 1B | This study |
| D354 | DUY326 transformed with pCMS154-HSP82 | Figure 1B | This study |
Acknowledgments
We are grateful to D. G. Drubin (University of California, USA), T.C. Huffaker (Cornell University, Ithaca, NY) and M.R. Culbertson (University of Wisconsin, USA) for providing the cct mutant strains. We thank J. L. Brodsky for the plasmids pFR22 and pCMS154-HSP82.
References
Funding
This work was supported by Council of Scientific and Industrial Research (CSIR), India [Grant No: 37(1571)/12/EMRII].
Reviewed By
AnonymousHistory
Received: June 27, 2021Revision received: July 29, 2021
Accepted: August 2, 2021
Published: August 5, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Narayanan, A; Kabir, MA (2021). Sorbitol and PKC1 overexpression alleviate temperature sensitivity in chaperonin mutants of Saccharomyces cerevisiae. microPublication Biology. 10.17912/micropub.biology.000440.Download: RIS BibTeX