Abstract
The establishment of cell polarity in eukaryotes involves the asymmetric distribution of messenger RNAs (mRNAs). In Saccharomyces cerevisiae, establishment of the cell polarity that gives rise to mother and daughter cells concurs with the selective targeting of more than 30 mRNAs toward the bud tip. Different mRNAs are segregated at different cell cycle stages, namely early during S phase, in a process dependent on anchoring to the endoplasmic reticulum (ER), or later in G2 or mitosis, in an ER-independent manner. In spite of this difference, this transport requires in all cases the Myo4p motor and its interaction with actin, the adaptor protein She3p and a third, RNA-binding protein docking this complex at the mRNA itself. This protein is universally considered to be She2p. Yet, the majority of mRNAs whose segregation was shown to be She2p-dependent are not S-phase segregated ones. In other processes aimed at establishing polarity, such as during pheromone-stimulated G1 arrest, the coupling of mRNAs to the ER during their transport is She2p-independent. We have therefore asked if the segregation to the bud of a model S-phase-specific mRNA, EAR1, is dependent on She2p or not. We report that a modest yet consistent percentage of EAR1 segregating particles achieves polarization without She2p. Our data invite to a re-evaluation of the absolute necessity for She2p for daughter cell-targeted mRNAs distribution.
Description
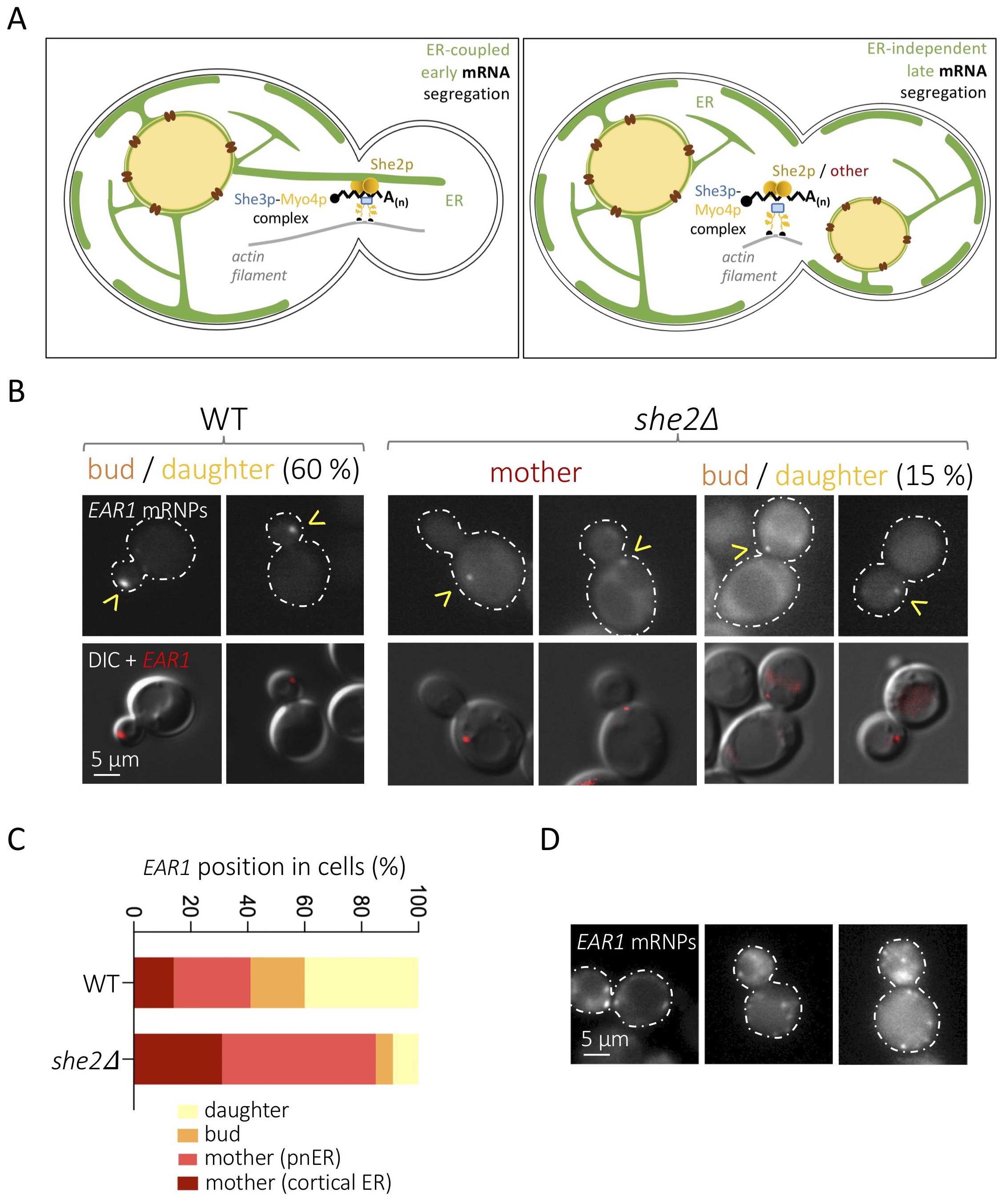
The establishment of cell polarity in eukaryotes involves the asymmetric distribution of messenger RNAs (mRNAs). In Saccharomyces cerevisiae, polarization leads to the budding of daughter cells, and more than 30 mRNAs subjected to selective targeting toward the bud tip have been characterized (Shepard et al. 2003; Aronov et al. 2007). Different mRNAs are segregated at different cell cycle stages, which is mostly dictated by their expression profiles. This way, mRNAs such as WSC2, IST2 or EAR1 are delivered during S / G2 phases, when the bud starts to emerge, while others, such as ASH1, are dispatched late during mitosis (Shepard et al. 2003; Fundakowski et al. 2012). Importantly, segregation of “early” mRNAs is coupled to, and dependent on, the simultaneous segregation of the endoplasmic reticulum (ER) toward the daughter cell (Figure 1A, left panel), while late-segregating mRNAs are not dependent on ER inheritance (Figure 1A, right panel) (Fundakowski et al. 2012). In both cases though, the trafficking of these mRNAs requests the myosin Myo4p, which promotes transport along actin filaments, and the adaptor protein She3p (Münchow et al. 1999) (Figure 1A). Using the mitosis-specific ASH1 mRNA as a model, a third, RNA-binding protein, She2p, was implicated as part of this “locasome” complex, its task being that of bridging the mRNA to the motor (Münchow et al. 1999; Bohl et al. 2000; Long et al. 2000; Takizawa and Vale 2000). Both the classical model ASH1 mRNA and 22 newly identified polarized mRNAs were found to be dependent on She2p for segregation (Bertrand et al. 1998; Shepard et al. 2003), and additional, asymmetrically distributed mRNAs, involved in polarity and exocytosis, were seen to co-fractionate with She2p (Aronov et al. 2007). Together, this has led to the general view that She2p is the universal factor docking mRNAs destined for the daughter cell at the Myo4p-She3p transporting complex. What is more, in the case of S-phase-segregating mRNAs, She2p is proposed to couple mRNA distribution and ER inheritance through its ER membrane-binding properties (Genz et al. 2013) (Figure 1A, left panel).
We realized that, of the 22 mRNAs whose asymmetric distribution has been demonstrated to rely on She2p, only 2 (DNM1 and WSC2) were early, S-phase-segregated (Shepard et al. 2003). Other early segregating mRNAs were shown to depend on She2p to associate to the ER, yet whether this is necessary to reach the bud was not evaluated (Aronov et al. 2007). We have therefore assessed, for the first time, the bona-fide “early segregating” mRNA EAR1, as defined by its dependence on ER inheritance (Fundakowski et al. 2012), for its dependency (or not) on She2p in order to be polarized. We transformed wild type (WT) and she2∆ S. cerevisiae cells with two vectors: one expressing the mCherry fluorophore fused to the coding sequence for the single-stranded RNA phage capsid protein MS2, and another plasmid expressing, from its own promoter, a modified EAR1 bearing 12 stem loops recognized by the MS2 protein (Bertrand et al. 1998). Cells growing exponentially in minimal medium selective for both vectors were imaged to observe the position of MS2-revealed EAR1 mRNA particles (mRNPs) (Figure 1B). We found that the absence of She2p substantially limits EAR1 delivery to the daughter cell, yet does not abolish it (Figure 1B,C). In particular, we observed that, compared to the 60% of MS2-defined EAR1 foci reaching the bud and the daughter in the WT in asynchronous cultures, only 15% did in she2∆ cells (Figure 1B,C). This is relevant, because a 15% delivery success is classified in the literature as weak yet existent targeting (Shepard et al. 2003). Further, defective mRNA particle delivery of concerned mRNAs in she2∆ cells has been described to concur with abundant, randomly distributed, tiny signals (Shepard et al.2003), something we never observed for EAR1 in she2∆ cells, and seldom in WT ones (Figure 1D).
As a whole, our data suggest that the segregation of some daughter-targeted mRNAs may occur in the absence of She2p. This remains a novelty because, up-to-date, delivery events are reported to be She2p-dependent (Bertrand et al. 1998; Shepard et al. 2003). It could be that additional factors, yet to be identified, are in charge of a subpopulation of these mRNAs (Figure 1A, right panel, “other”). It is indeed tempting to imagine that some bud-targeted mRNAs are not linked to the Myo4p-She3p complex and actin filaments through She2p. There is evidence for the existence of alternative dockers, as is the case for Scp160p, which fulfils this function during the polarized delivery of mRNAs to the shmoo tip in G1 phase in response to sexual pheromones (Gelin-Licht et al. 2012). But it could also be argued that the events in which we detected EAR1 in the daughter cell are due to passive diffusion. Even if random MS2 spots have been reported to distribute as multiple, small, disordered foci (Bertrand et al. 1998), which is not what we detect (Figure 1D), this is not enough to dismiss the possibility that EAR1 particles manage to reach the daughter cell passively. Since She2p is needed to anchor the targeted mRNA to the early segregating ER, it is likely that EAR1 She2p-independent distribution to the daughter occurs later in the cell cycle thanks to the prolonged window of time before division. Last, it could also be argued that the MS2-mCherry tagging supports artefactual association of molecules, thus favouring their segregation. Although possible, this is unlikely, as MS2-driven and in-situ hybridization-mediated mRNAs detection proves comparable (Schmid et al. 2006). In all instances, our work thus establishes a precedent justifying the careful assessment of other early bud-segregated mRNAs for their (in)dependence on She2p, or on alternative dockers.
Methods
Request a detailed protocolSaccharomyces cerevisiae cells were grown at 25°C in selective YNB liquid medium supplemented with 2% glucose without histidine and uracil to ensure plasmid maintenance. All experiments were performed with exponentially growing cells. For microscopy analyses, 1 mL of the culture of interest was centrifuged; then, the supernatant was thrown away and the pellet was resuspended in the remaining 50 μL. Next, 3 μL of this cell suspension was directly mounted on a coverslip for immediate imaging of MS2-mCherry signals. Fluorescent signals were detected using the adequate wavelength and acquired with a Zeiss Axioimager Z2 microscope and Metamorph software. Subsequent image visualization and analysis were performed with Image J v2.0.0-rc-69/1.52i. The determination of the sub-cellular localization of EAR1 signals in all cells displaying any was done through visual inspection by the experimenter. GraphPad Prism was used to plot the results.
Reagents
The wild-type strain (MM-35) is a W303 strain corrected for the RAD5 gene and the she2∆ strain (MM-302) was built by classical gene disruption using the G418 resistance cassette kanMX6. Plasmids RJP1815 (pYCplac33-EAR1-12MS2-URA3) and RJP1889 (pCP-MS2-5xmCherry-HIS3) were a kind gift from Pr. Ralf-Peter Jansen, Tübingen University. We also used DAPI (D9542, Sigma-Aldrich).
Acknowledgments
We thank Pr. Ralf-Peter Jansen for the gift of the RJP1815 and RJP1889 plasmids. We acknowledge the imaging facility MRI, a member of the national infrastructure France-BioImaging, supported by the French National Research Agency (ANR-10- INBS-04, Investissements d’avenir).
References
Funding
This research was funded by the ATIP-Avenir program, La Ligue contre le Cancer et l’Institut National du Cancer (PLBIO19-098 INCA_13832), France.
Reviewed By
Manuel MendozaHistory
Received: August 6, 2021Revision received: September 3, 2021
Accepted: September 7, 2021
Published: September 13, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Samardak, K; Moriel-Carretero, M (2021). Daughter cell-targeted mRNAs can achieve segregation without the universal Endoplasmic Reticulum docker She2p. microPublication Biology. 10.17912/micropub.biology.000458.Download: RIS BibTeX