Centre de Recherche en Biologie cellulaire de Montpellier (CRBM), Université de Montpellier, Centre National de la Recherche Scientifique, 34293 Montpellier CEDEX 05, France
Abstract
The endoplasmic reticulum (ER) is a central organelle in charge of correct protein folding; lipids synthesis, modification, and sorting; as well as of maintenance of calcium homeostasis. To accomplish these functions, the ER lumen possesses an oxidative potential. Challenging cells with reductive agents therefore provokes an ER stress that immediately affects protein folding, and which morphologically manifests by an expansion of the cytoplasmic ER network. Yet less is known about the impact on the ER of exposing cells to oxidative agents, which risk to exacerbate the basal, physiologically oxidative environment. We have monitored the morphology of the ER of Saccharomyces cerevisiae in response to this type of treatment. We bring the notion that oxidative agents give rise to diverse alterations in the perinuclear ER subdomain that are suggestive of lipid metabolism perturbations.
Description
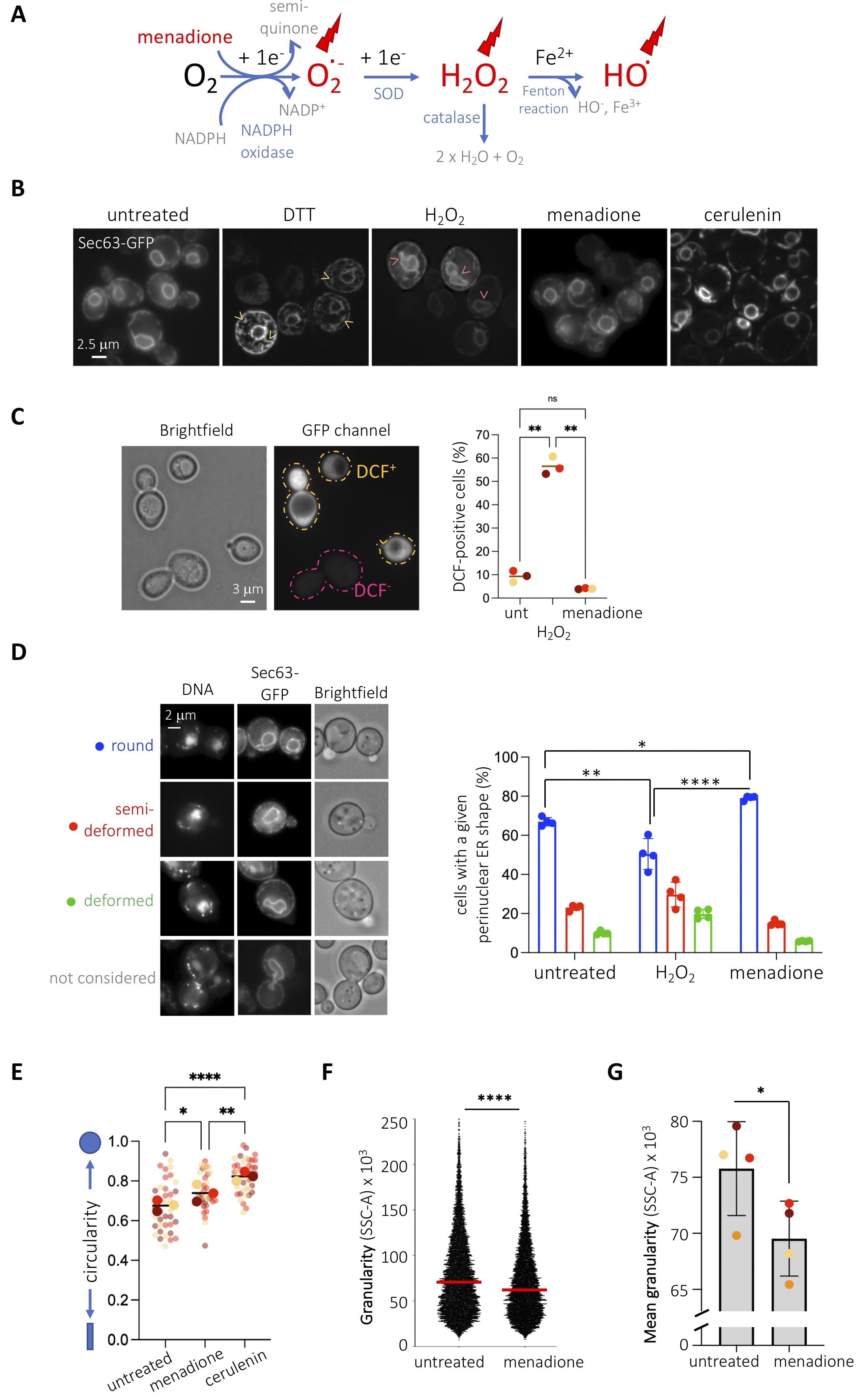
Our laboratory studies the relationship between genotoxic stress and the endoplasmic reticulum (ER). Since multiple genotoxins are reported to generate oxidative stress (Mizumoto et al. 1993; Singh and Xu 2016), we wanted to establish the impact of bona-fide oxidative agents on basal ER morphology as a control before further studies. To this end, we chose two agents, menadione and hydrogen peroxide, whose action mode relies on different reactive oxygen species (Figure 1A and (Castro et al. 2008; Roscoe and Sevier 2020)). We visualized the ER of Saccharomyces cerevisiae cells by transforming them with a plasmid expressing the transmembrane ER protein Sec63p tagged with GFP at its C-terminus (Prinz et al. 2000). Under basal conditions, this tool permits to see a central ring, the perinuclear ER (also known as the outer nuclear membrane), the cortical ER under the plasma membrane and eventual connections between both, the cytoplasmic ER (Figure 1B). We treated cells with the reducing agent dithiothreitol (DTT) to trigger a bona-fide unfolded protein ER stress which, as expected, gave rise to an overgrowth of cytoplasmic ER membranes, a response aimed at increasing the challenged ER protein folding capacity (Ron and Walter 2007). When cells were exposed to 10 mM hydrogen peroxide, whose entry in the cells was validated by an increase in fluorescence when using the probe 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) (Figure 1C), we did not find any alteration in the cytoplasmic ER, but a deformation in the perinuclear ER subdomain (Figure 1B). Alterations in shape at the perinuclear ER are suggestive of lipid rather than protein ER stress (Santos-Rosa et al. 2005; Witkin et al. 2012). To quantify this phenomenon, we established three degrees of deformation, which transitioned from almost perfectly spherical, to semi-deformed, to deformed (Figure 1D). This classification intentionally excludes cells in G2 / M, for which the nuclear membrane appears distorted because of cell division (Figure 1D). In comparison with the untreated condition, in which a 65% of cells display round nuclear shapes, hydrogen peroxide made this value significantly decrease down to 50% (Figure 1B & 1D, right). Next, we exposed cells to menadione, whose oxidative effect, mainly exerted by superoxide anions (Figure 1A), was also indirectly validated using the probe 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) (Figure 1C). Menadione also triggered a modification exclusively at the perinuclear ER but, in striking contrast, it was integrally in the opposite direction than hydrogen peroxide: the perinuclear ER displayed a perfectly spherical shape in 80% of the population (Figure 1B & 1D, right). This phenomenon mimics the effect of cerulenin (Figure 1B), an inhibitor of fatty acid synthase (Inokoshi et al. 1994) in whose presence phospholipid synthesis decreases thus nuclear membranes are incapable of expansion (Schneiter et al. 1996; Yam et al. 2011). In agreement, we confirmed that cells treated with menadione or cerulenin possess nuclei with increased circularity (Figure 1E). For the same reason, cerulenin induces the consumption of lipid stores, thus decreasing the number of lipid droplets in S. cerevisiae (Jacquier et al. 2011). To reinforce the notion that menadione limits lipid biosynthesis, we measured the granularity of cells as a readout for their content in lipid droplets and membranes (Suzuki et al. 1991; Lee et al. 2004). In agreement, we found that menadione decreases cell granularity, as established by flow cytometry (Figure 1F), and this in a very reproducible manner (Figure 1G).
In sum, we report that two oxidative stressors elicit morphological changes in the ER reminiscent to lipid rather than unfolded protein stress. Yet, hydrogen peroxide reactive oxygen species trigger nuclear membrane expansion or deformation while superoxide anion species match an extremely circular morphology, suggestive of an anti-lipogenic phenotype. This is in striking agreement with a recent report in which menadione was described to exert an anti-adipogenic activity by repressing the expression of multiple enzymes involved in the lipogenic program, among which fatty acid synthase (Funk et al. 2021). Our data therefore set the bases to further explore the molecular link between oxidative and lipid ER stresses.
Methods
Request a detailed protocolSaccharomyces cerevisiae cells were grown at 25°C in selective YNB liquid medium supplemented with 2% glucose in the absence of uracil to ensure plasmid maintenance. All experiments were performed with exponentially growing cells. To acquire side-scattered light values by flow cytometry, 430 μL of culture samples at 107 cells/mL were fixed with 1 mL of 100% ethanol. Cells were centrifuged for 1 minute at 16000g and resuspended in 500 μL 50 mM Na-Citrate buffer containing 5 μL of RNase A (10 mg/mL, Euromedex, RB0474) for 2 hours at 50°C. 6 μL of 20 mg/mL Proteinase K (Euromedex, EU0090-C) were added for 1 hour at 50°C. Aggregates of cells were dissociated by brief sonication. 20 μL of this cell suspension were incubated with 200 μL of 50 mM Na-Citrate buffer containing 4 μg/mL Propidium Iodide (FisherScientific). Data were acquired on a MACSQuant Analyser X (Miltenyi Biotec) and analyzed with FlowJo software. For microscopy analyses, 1 mL of the culture of interest was centrifuged; then, the supernatant was thrown away and the pellet was resuspended in the remaining 50 μL. Next, 3 μL of this cell suspension was directly mounted on a coverslip for immediate imaging of Sec63p GFP-tagged protein signals. To measure cellular levels of hydrogen peroxide, cells were incubated with 10 µM H2DCFDA for 1 h prior to harvesting and washed twice with 1x PBS prior to imaging. Fluorescent signals were detected using the adequate wavelength and acquired with a Zeiss CCD AxioCam MRm monochrome camera from a Zeiss AxioImager Z1 microscope with ApoTome technology using a 40× Plan Apochromat 1.3-NA oil objective lens and Zen software. Images were acquired at 20–23 °C. Subsequent image visualization and analysis were performed with Image J v2.0.0-rc-69/1.52i. The determination of the percentage of different ER configurations was done through visual inspection by the experimenters. To quantify circularity of nuclei, Sec63p-GFP images were thresholded using the algorithm Otsu in ImageJ, and circularity measured using the command “shape descriptors”. Visual inspection by the experimenter of selected particles compared to the original images ensured that only real nuclei were considered. GraphPad Prism was used to plot and statistically analyze the results.
Reagents
The wild-type strain (MM-35) is a W303 strain corrected for the RAD5 gene. We used cerulenin (SC-200827A, Santa Cruz Biotechnology), DAPI (D9542, Sigma-Aldrich), DTT (10197777001, Sigma-Aldrich), menadione (M5750-25G, Sigma-Aldrich), H2O2 (H1009, Sigma-Aldrich) and H2DCFDA (35845, Sigma-Aldrich). pJK59 (CEN-URA3-SEC63promoter-SEC63-GFP(S65T,V163A)), used to visualize the ER, was a kind gift from Dr. Sebastian Schuck.
Acknowledgments
We thank Philippe Pasero for hosting the execution of these experiments and for his unconditional support to the research in our laboratory. We are grateful to Sebastian Schuck for the gift of the pSec63p-GFP plasmid. We acknowledge the imaging facility MRI, a member of the national infrastructure France-BioImaging, supported by the French National Research Agency (ANR-10- INBS-04, Investissements d’avenir).
References
Funding
This research was funded by the For Women in Science Fondation L’Oréal-UNESCO program, the ATIP-Avenir program, La Ligue contre le Cancer et l’Institut National du Cancer (PLBIO19-098 INCA_13832), France.
Reviewed By
AnonymousHistory
Received: July 16, 2021Revision received: September 3, 2021
Accepted: September 10, 2021
Published: September 20, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Torán-Vilarrubias, A; Moriel-Carretero, M (2021). Oxidative agents elicit endoplasmic reticulum morphological changes suggestive of alterations in lipid metabolism. microPublication Biology. 10.17912/micropub.biology.000462.Download: RIS BibTeX