Abstract
Phase separation has emerged as a widespread process of organizing the cytoplasm of diverse eukaryotic cells. In C. elegans oocytes, several RNA binding proteins are condensed into germ granules called P granules. Prior studies studying the phase transitions of RNA binding proteins in response to increased temperature have suggested that PGL-1 decondenses in oocytes in response to heat. Here, we confirm this finding with a new reporter strain and demonstrate the sensitivity of PGL-1 to temperature changes.
Description
In many cell types, subsets of RNA binding proteins adopt a default de-mixed state, condensing into ribonucleoprotein (RNP) granules (Alberti and Carra, 2018). In the C. elegans germ line PGL-1, an RGG domain protein, is condensed into P granules. P granules associate closely with nuclear pores of pachytene nuclei in the adult germ line but detach from nuclei as oocytes develop (Strome and Wood, 1982; Pitt et al., 2000) and contribute to maternal mRNA regulation (Voronina, 2013). In contrast, other RNA binding proteins have a default mixed, or decondensed, state in the adult germ line. For example, the KH domain protein MEX-3 is largely diffuse throughout the cytosol of oocytes and condenses into P granules in early embryos (Draper et al., 1996).
Multiple studies have shown that several types of RNA binding proteins undergo dynamic phase separation during certain environmental stresses and extended meiotic arrest. P-granule proteins, such as PGL-1, P-body proteins such as CGH-1, and stress granule proteins such as TIAR-2 are all detected in large RNP granules in meiotically arrested oocytes (Jud et al., 2008; Noble et al., 2008; Wood et al., 2016). The RNP granules are hypothesized to maintain oocyte quality during delays in fertilization. Similarly, in a study of heat stress responses, P-body and stress granule proteins also condense into large RNP granules in oocytes (Jud et al., 2008). In contrast, in the same study a summary table states that PGL-1 granules are not detected after heat stress; however, images of this experiment were not published (Jud et al., 2008).
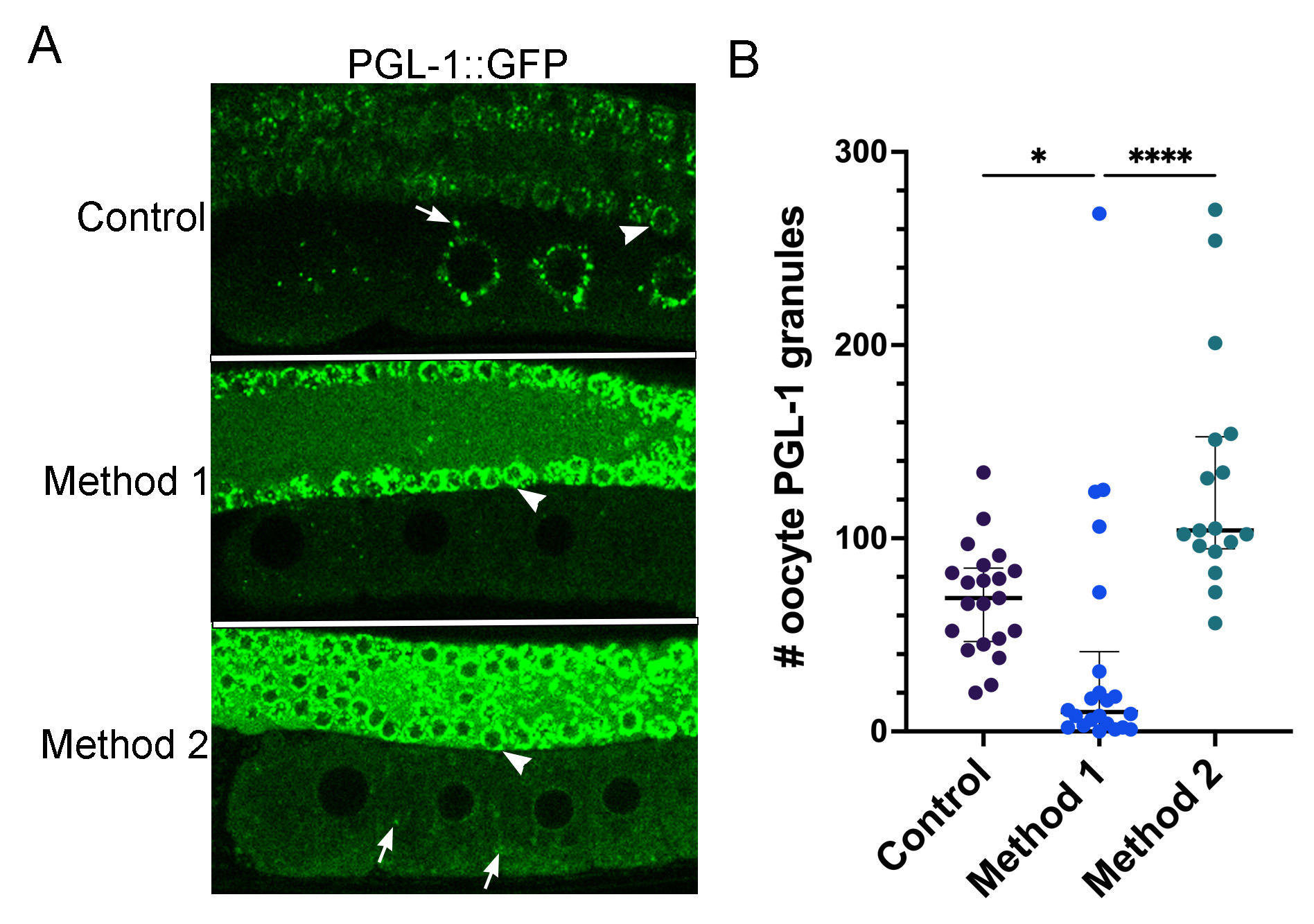
Recent studies using the PGL-1::GFP reporter strain demonstrate that PGL-1 decondenses rapidly in the early embryo after the temperature is upshifted to 34°C (Putnam et al., 2019). P granules in the early embryo are similar, but not identical to P granules in oocytes. As mentioned above, a subset of proteins are transient P granule components. Since MEG-3 plays an important role in stabilizing PGL-1 granules in the early embryo, but does not associate strongly with P granules prior to fertilization, it is not necessarily the case that P granule dynamics in oocytes will recapitulate dynamics observed in embryos (Wang et al., 2014). The prior heat stress study by Jud et al., was conducted before CRISPR reporter strains were available; therefore, we asked if PGL-1::GFP decondenses in oocytes in response to increased temperature. PGL-1 was detected in perinuclear and cytoplasmic P granules as expected in control oocytes (median of 69 granules) and in the distal germ nuclei. In contrast, after 40 minutes at 34°C in a stagetop incubator (Method 1), in the majority of worms PGL-1 appeared dispersed, with significantly fewer granules detected in the proximal -2 to -5 oocytes (median of 10 granules) (Figure 1). This result appears similar to the original result described, but not shown, that detected PGL-1 by immunofluorescence after fixing worms immediately after being exposed to 34°C via a standard incubator (Jud et al., 2008). As a side note, we did not detect similar dispersal of PGL-1 in the distal nuclei, suggesting nuclear-associated PGL-1 granules may be less sensitive to temperature changes (arrowheads, Figure 1). While this manuscript was in revision, an independent study found similar results in oocytes and distal nuclei (Fritsch et al., 2021). Interestingly, when we incubated the PGL-1:GFP worms in a standard incubator and imaged without a stagetop incubator (Method 2), many more PGL-1 granules were detected in oocytes than with Method 1 (median of 104 granules; Figure 1). This may not seem surprising since the worms could be expected to recover at ambient temperature, and many phase transitions are reversible. However, it is notable, as a practical consideration, that the phase separation of PGL-1 into granules occurred quickly, within 15 minutes at ambient temperature.
From these results with the PGL-1::GFP strain, we confirm that in proximal oocytes PGL-1 is extremely sensitive to temperature changes, and behaves as a dynamic, liquid-like protein, similar to its behavior in the early embryo (Jud et al., 2008; Putnam et al., 2019). While short fluctuations in temperature during heat stress experiments may not adversely affect all fluorescent reporter strains, they can clearly affect phase transitions of liquid-like proteins such as PGL-1.
Methods
Request a detailed protocolHeat Stress
Method 1. The Tokai Hit stage top incubator was used at 34°C with the Nikon A1R laser scanning confocal microscope. Young adult PGL-1::GFP worms were placed on small NGM plates and incubated in the stage top incubator for 40 minutes at 34°C. Within two minutes at room temperature, the worms were transferred to a 2% agarose pad prior to imaging at 34°C. The temperature remained at 34°C throughout imaging, and imaging was completed within 15 minutes.
Method 2. NGM plates with young adult PGL-1::GFP worms were placed in a standard incubator at 34°C for 40 minutes. Worms were transferred to a 2% agarose pad and imaged at ambient temperature on the Nikon A1R confocal microscope. Worms were at ambient temperature for 8-15 minutes before images were acquired.
Microscopy
Worms were mounted on a 2% agarose pad and imaged in 6.25mM levamisole to immobilize worms, or 2.5mM levamisole to prevent bursting of heat-stressed worms. Imaging was performed using a Nikon A1R confocal microscope using a 60x N.A. 1.2 water-immersion objective. 0.5mM Z-slices were collected. Images were pseudo-colored using Adobe Photoshop.
Statistical Analyses
ImageJ was used to determine the number of granules in the mid-focal Z-slices of the most proximal (-2 to -5) oocytes. An ANOVA/ Kruskal Wallis test was conducted using GraphPad Prism v.8 to analyze statistical significance. GPower 3.1 was used to conduct a power analysis to determine the sample size.
Reagents
| Strain | Genotype | Available from |
| JH3269 | pgl-1(ax3122[pgl-1::gfp]) IV | CGC |
Acknowledgments
We appreciate the Schisa lab members, and Xantha Karp for their helpful comments on this manuscript.
References
Funding
This work was supported by the National Institute of Health grant 2R15GM109337 (to J.S.) and a CMU College of Science and Engineering research assistantship (to B.W.). B.W. is a trainee in the CMU Biochemistry, Cell and Molecular Biology program.
Reviewed By
AnonymousHistory
Received: August 26, 2021Revision received: September 14, 2021
Accepted: September 21, 2021
Published: September 24, 2021
Copyright
© 2021 by the authors. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Citation
Watkins, B; Schisa, JA (2021). Phase separation dynamics of the C. elegans PGL-1 P granule protein in oocytes are sensitive to heat stress. microPublication Biology. 10.17912/micropub.biology.000476.Download: RIS BibTeX